Figure 6.
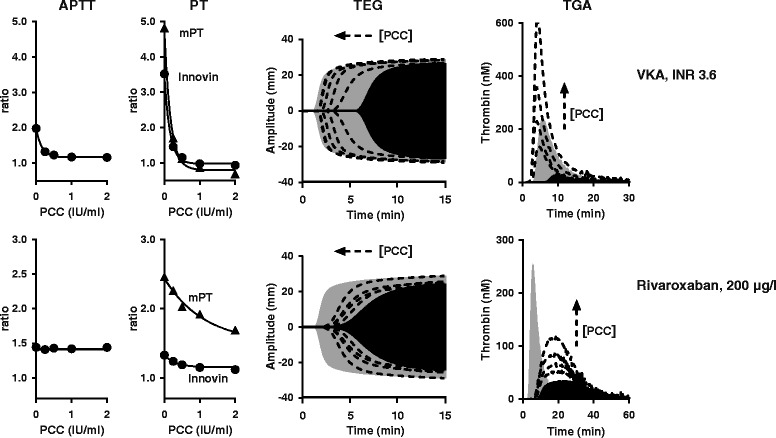
In vitro reversal of VKA- and rivaroxaban anticoagulation by PCC. VKA anticoagulated plasma (George King Biomedical Inc) and normal plasma anticoagulated with 200 μg/L rivaroxaban was spiked with increasing PCC dose (4-factor PCC, Cofact, Sanquin, Amsterdam, The Netherlands). APTT, PT, TEG and TGA was performed as described [32,68]. APTT was with reagent Actin FSL from Siemens Healthcare Diagnostics. PT was with Innovin (Siemens). The modified PT (mPT) reagent was prepared by mixing 1 volume Thromborel S (Siemens Healthcare Diagnostics) with 1.25 volumes 80 mM CaCl2. TEG was with 4 μM phospholipids (Rossix AB) and 10 pM TF (Innovin, Diagnostica Stago). TGA was with 4 μM phospholipids and 5 pM TF (PPP reagent, Thrombinoscope). Filled grey TGA and TEG curves: normal plasma, filled black curves: anticoagulated plasma without spiked PCC, dotted lines: anticoagulated plasma with increasing PCC dose (0, 0.25, 0.5, 1, 2 IU/ml). Remarkable feature for NOAC reversal is that the response to PCC is strongly TF concentration dependent; at a high TF concentration, less PCC is needed to restore TGA-peak and TGA-AUC [32,68]. 1 IU/ml PCC ≈ 40 IU per kg body weight.
