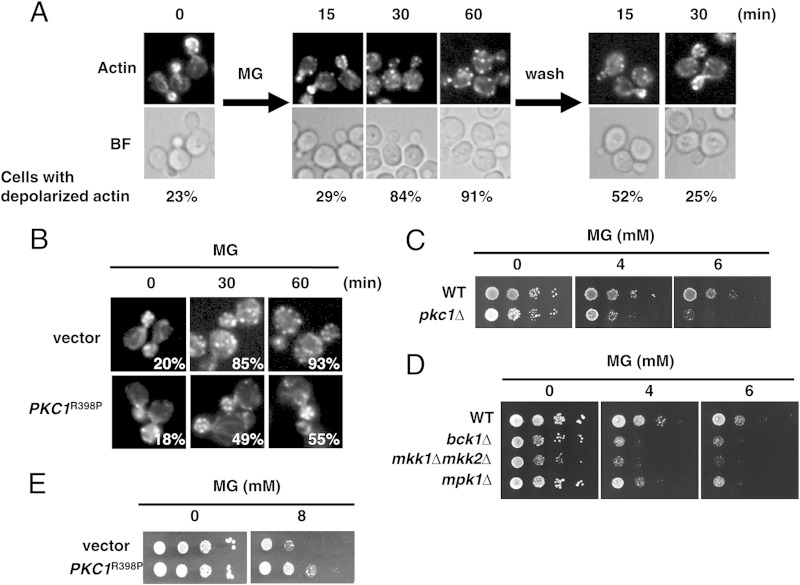
FIG 1.
MG depolarizes the actin cytoskeleton. (A) Cells (YPH250) were cultured in SD medium until the A610 was 0.3 and were then treated with 10 mM MG for 60 min. Cells were stained for actin with rhodamine-phalloidin and observed using a fluorescence microscope. The proportion of cells with depolarized actin was determined by counting the cells in which actin had not accumulated in the bud. More than approximately 200 cells were counted in each experiment. BF, bright field. (B) Cells (YPH250) carrying YCp50 (vector) or YCp50-PKC1R398P were cultured in SD medium until the A610 was 0.3 and were then treated with 10 mM MG for the times indicated. Cells were stained for actin with rhodamine-phalloidin. The proportion of cells with depolarized actin is indicated for each image. (C) Wild-type (DL100) and pkc1Δ (DL376) cells were cultured in SD medium containing 0.5 M sorbitol until the log phase of growth and serially diluted (1:10) with 0.5 M sorbitol solution. An aliquot (4 μl) of each cell suspension was spotted onto SD agar plates containing 0.5 M sorbitol with or without MG. (D) Cells (YPH250) defective in the components of the Mpk1 MAP kinase cascade (bck1Δ, mkk1Δ mkk2Δ, and mpk1Δ cells) were cultured in SD medium until the log phase of growth and serially diluted (1:10) with 0.85% NaCl solution, and 4 μl of each cell suspension was then spotted onto SD agar plates containing MG. (E) Cells (YPH250) carrying YCp50 (vector) or YCp50-PKC1R398P were cultured in SD medium until the log phase of growth. The cell suspension was diluted as described in the legend to panel D, and 4 μl of each suspension was spotted onto SD agar plates containing MG.