Abstract
Background
Optimal surgical management of patients with invasive lobular carcinoma (ILC) who undergo neoadjuvant chemotherapy (NAC) is unknown. We evaluated optimal margin distance and local recurrence (LR) rates for these patients.
Methods
Ninety three (30%) of 311 patients with ILC received NAC. We examined margin status, residual disease after re-excision and clinical outcomes.
Results
Margin positivity rates after the final operative procedure was similar between the NAC and surgery first group (P>.05). The proportion of patients, stratified by margin status, who were taken back for re-excision was not different between the 2 groups and similarly there were no differences in frequency of residual disease (all P>.05). At a median follow-up of 3.1 years, one patient in the NAC group and 2 in the surgery first group developed LR (p=1.0).
Conclusion
Patients with ILC who have undergone NAC and have margins >1mm have a low probability of residual disease and LR.
Keywords: breast, lobular cancer, margins, neoadjuvant chemotherapy, local recurrence
Introduction
Invasive lobular carcinoma (ILC) is composed of non-cohesive cells individually dispersed or arranged in single-file linear pattern in a fibrous stroma 1. There has been a continuous increase in the incidence of this disease in women over 50 throughout the last 20 years, now representing 5–15% of invasive breast tumors 1. Due to the insidious infiltrative nature of ILC, majority of patients present at a more advanced stage with an ill-defined mass 1, 2. Breast conservation therapy (BCT) as the primary surgical treatment for ILC has been controversial. Singletary et al reported from the National Cancer Data Base that there was no increase in local recurrence rates for patients with early stage ILC treated with breast conservation surgery, defined as pathologic T1 or T2 pure ILC, compared to patients undergoing mastectomy 3. Furthermore, Bouvet et al assessed margin status in relation to local-regional recurrence (LR) in patients with early stage ILC 4. The majority of their patients (69%) had pathologic T1 tumors and they noted that patients with a positive or close margin (≤1mm) were at increased risk for LR. Margin status was the only factor determined to influence the potential risk for LR on univariate analysis. In addition, they found a low rate of LR (6%) at 5 years in patients with a negative margin (>1mm). Other investigators have reported that patients with early stage ILC have similar local recurrence and overall survival rates to patients with early stage invasive ductal carcinoma when treated with BCT 5–8.
For ILC patients with more advanced disease at presentation, neoadjuvant chemotherapy may be offered to decrease tumor size and allow for BCT. Optimal margin width in this setting is unknown. We performed a retrospective study to determine the effect of neoadjuvant chemotherapy on the assessment of margin status in order to better define the optimal margin width in the more advanced stage ILC patient, with the ultimate goal to minimize risk of local recurrence.
Methods
Patient population
Patients diagnosed with pure invasive lobular carcinoma of the breast from April 1998 to September 2006 were identified from the University of Texas, M.D. Anderson Cancer Center Surgical Pathology database. Records were reviewed and relevant clinicopathological features extracted for analysis. Patients were divided into two subgroups; 1) those who had undergone neoadjuvant chemotherapy who formed the study population (n=93), 2) a control cohort who did not undergo neoadjuvant chemotherapy (n=218). At M.D. Anderson Cancer Center, patients presenting with nodal disease and/or T2 or larger primary tumors are considered eligible for neoadjuvant chemotherapy. Approval for the study was obtained from the M.D. Anderson Cancer Center Institutional Review Board.
Surgical treatment and margin status
All patients underwent surgical treatment, either breast conservation therapy or mastectomy, for their primary tumor. The decision to proceed with mastectomy was based on presence of multicentric disease, large primary tumors relative to breast size as well as patient preference. Pathological margins were reviewed and patients were classified into two groups according to the distance between tumor and the margin of resection; “positive” (grossly positive margin or tumor ≤1mm from the margin) or “negative” (tumor >1mm from the resection margin). The presence or absence of residual disease, which was defined as ILC or ductal carcinoma in situ, was determined for all patients undergoing re-excision. We opted to exclude LCIS given the preponderance of the data that shows presence of LCIS at margins of resection does not increase the probability of local recurrence 9, 10. Re-excision was classified as either a second operative procedure or additional margins taken at the time of the initial operation.
Statistical methods
All calculations were performed using SAS 9.0. Chi-Square test or Fisher’s exact test was used to assess the association between two categorical variables. P-values of less than or equal to 0.05 were considered to be statistically significant.
Results
Patient and tumor characteristics
Three hundred eleven patients with a diagnosis of pure ILC who had undergone surgical treatment at M.D. Anderson Cancer Center were identified. Ninety-three (30%) of these patients received neoadjuvant chemotherapy. Patient and tumor characteristics are listed in Table 1. The median age at presentation in the neoadjuvant and the surgery first groups was 54 years (range 35–86) and 60 years (range 29–91) respectively (p<0.01). As would be expected, there was a significant difference in the clinical T stage between the two groups with the majority of patients (51.6%) in the neoadjuvant group presenting with clinical T2 tumors compared to the majority of patients (59.6%) in the surgery first group presenting with clinical T1 tumors (p<0.01). Twenty-eight of the 93 patients (30.1%) in the neoadjuvant group underwent breast conservation therapy for their first surgical procedure while 65 of the 93 patients (69.9%) underwent mastectomy. In contrast, in the surgery first group there was an almost even distribution between BCT and mastectomy (49.1% and 50.9% respectively). These differences in BCT rates between the two patient cohorts were magnified after re-excision with only 16 patients (17.2%) in the neoadjuvant group successfully completing breast conservation therapy compared to 92 of the 218 patients (42.2%) who underwent BCT as their final surgery in the surgery first group (p<0.01).
Table I.
Patient and tumor characteristics
| Neoadjuvant Chemotherapy n=93 | Surgery first n=218 | p-value | |
|---|---|---|---|
|
| |||
| Age | p<0.01 | ||
| Range | 35 – 86 | 29 – 91 | |
| Median | 54 | 60 | |
| Clinical T Stage | p<0.01 | ||
| T1 | 11 (11.8%) | 130 (59.6%) | |
| T2 | 48 (51.6%) | 70 (32.1%) | |
| T3 | 25 (26.9%) | 17 (7.8%) | |
| T4 | 9 (9.7%) | 1 (0.5%) | |
| Clinical N Status | p<0.01 | ||
| N0 | 42 (45.2%) | 197 (90.3%) | |
| N1 | 40 (43.0%) | 20 (9.2%) | |
| N2 | 2 (2.1%) | 0 (0.0%) | |
| N3 | 9 (9.7%) | 1 (0.5%) | |
| ER/PR Status | p=0.26 | ||
| ER positive/PR positive | 57 (62.0%) | 142 (66.7%) | |
| ER positive/PR negative | 27 (29.4%) | 63 (29.6%) | |
| ER negative/PR positive | 2 (2.2%) | 1 (0.5%) | |
| ER negative/PR negative | 6 (6.5%) | 7 (3.3%) | |
| First Surgical Procedure | p<0.01 | ||
| BCT | 28 (30.1%) | 107 (49.1%) | |
| Mastectomy | 65 (69.9%) | 111 (50.9%) | |
| Final Surgical Procedure | P<0.01 | ||
| BCT | 16 (17.2%) | 92 (42.2%) | |
| Mastectomy | 77 (82.8%) | 126 (57.8%) | |
| Median Follow Up | p<0.01 | ||
| Years | 4 | 2.8 | |
Margin status
We first examined the difference in the margin status between the two groups at the time of initial excision, and then again after the final surgical procedure. For the entire cohort of patients receiving neoadjuvant chemotherapy, we found 30.1% (n=28) of patients had a positive margin after the initial surgery. This was statistically similar to the patients in the surgery first group who had a 28.4% (n=62) incidence of positive margins after their initial surgery. Thus, negative margins were achieved at the initial operation in 69.9% (n=65) of patients in the neoadjuvant group and 71.6% (n=156) of patients in the surgery first group (p=0.77) (Table 2).
Table II.
Initial vs final margin status among all patients
| Margin Status | Neoadjuvant Chemotherapy n=93 | Surgery first n=218 | p-value |
|---|---|---|---|
|
| |||
| Initial Excision | 0.77 | ||
| positive | 28 (30.1%) | 62 (28.4%) | |
| negative | 65 (69.9%) | 156 (71.6%) | |
| Final Excision | 0.49 | ||
| positive | 9 (9.7%) | 16 (7.3%) | |
| negative | 84 (90.3%) | 202 (92.7%) | |
After the final surgical procedure, 9.7% (n=9) of patients in the neoadjuvant group had a positive margin. This was comparable to the 7.3% (n=16) of patients in the surgery first group with a positive margin. Hence, 90.3% (n=84) and 92.7% (n=202) of patients were found to have a negative margin after final excision in the neoadjuvant and surgery first groups respectively (Table 2) (p=0.49).
We next evaluated margin status in the two groups as a function of the type of initial surgical procedure performed. Here we found that among patients that underwent BCT as their initial procedure, 71.4% (n=20) in the neoadjuvant group had a positive margin, compared to only 43.9% (n=47) in the surgery first group (Table 3). This difference was statistically significant (p=0.01). However, there was no difference in positive margin rates between the two groups of patients who had a mastectomy as the initial procedure with 12.3% (n=8) and 13.5% (n=15) of patients with a positive margin in the neoadjuvant and surgery first group respectively (p=0.8) (Table 3). Since the majority of patients in the neoadjuvant group had mastectomy as their first procedure, this likely accounts for the lack of overall difference in margin positive rates between the groups after initial excision (Table 2).
Table III.
Margin status after initial surgical procedure
| Neoadjuvant | Surgery first | p-value | |
|---|---|---|---|
|
| |||
| BCT* | |||
| positive | 20 (71.4%) | 47 (43.9%) | 0.01 |
| negative | 8 (28.6%) | 60 (56.1%) | |
| Neoadjuvant | Surgery first | p-value | |
|---|---|---|---|
|
| |||
| Mastectomy | |||
| positive | 8 (12.3%) | 15 (13.5%) | 0.80 |
| negative | 57 (87.7%) | 96 (86.5%) | |
BCT: breast conservation therapy
When these variables were re-examined for final procedure and final margin status, 18.8% (n=3) of patients in the neoadjuvant group who had BCT were found to have a positive margin in comparison to 8.7% (n=8) in the surgery first group (p=0.21) (Table 4). Similarly, there was no significant difference between the two groups with respect to those who underwent mastectomy for the final procedure with 92.2% (n=71) of patients in the neoadjuvant group and 93.6% (n=118) of patients in the surgery first group having a final negative margin (p=0.69) (Table 4).
Table IV.
Margin status after final surgical procedure
| Neoadjuvant | Surgery first | p-value | |
|---|---|---|---|
|
| |||
| BCT* | |||
| positive | 3 (18.8%) | 8 (8.7%) | 0.21 |
| negative | 13 (81.2%) | 84 (91.3%) | |
| Neoadjuvant | Surgery first | p-value | |
|---|---|---|---|
|
| |||
| Mastectomy | |||
| positive | 6 (7.8%) | 8 (6.4%) | 0.69 |
| negative | 71 (92.2%) | 118 (93.6%) | |
BCT: breast conservation therapy
Re-excision rates and residual disease
We wanted to determine whether neoadjuvant chemotherapy affected the frequency of finding additional disease at re-excision. Therefore, we next looked at the probability of finding additional residual disease among those patients that underwent re-excision.
We found that 82.1% (n=23) of patients in the neoadjuvant group with a positive margin underwent re-excision. Of these, 65.2% (n=15) had residual disease present (Figure 1). Similarly the majority of patients in the surgery first group with positive margins (79.0%, n=49) underwent re-excision. Although not statistically significant (p=0.15), slightly fewer patients, 46.9% (n=23), had residual disease present in the surgery first cohort versus the neoadjuvant group.
Figure I.
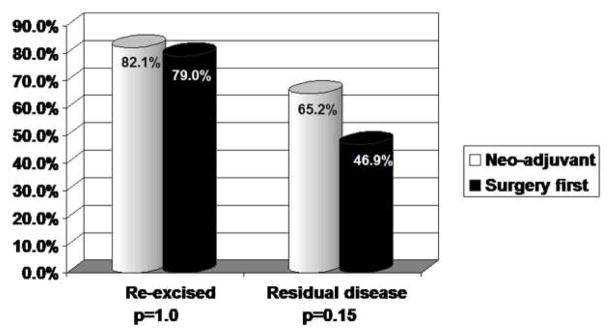
Re-excision rates and frequency of residual disease in patients with a positive margin at their initial surgery.
A small proportion of patients in both groups of the study underwent re-excision after an initial surgical procedure with negative margins, (18.5% and 25.6%, p=0.25) (Figure 2). Within this subpopulation, 2 (16.7%) patients in the neoadjuvant group had residual disease compared to 4 (10.3%) patients in the surgery first group (p=0.62).
Figure II.
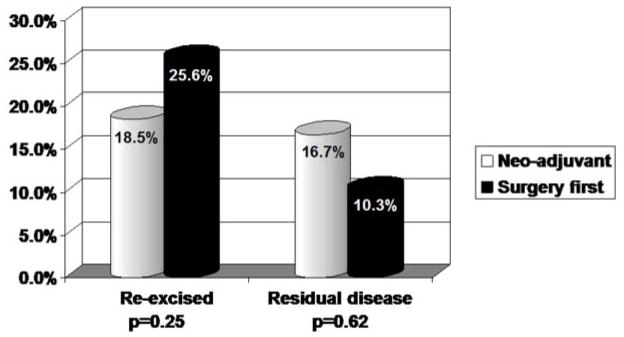
Re-excision rates and frequency of residual disease in patients with a negative margin at their initial surgery.
Local-regional recurrence
With an overall median follow-up of 3.1 years, three patients (1%) in this study have developed local-regional recurrence. Final surgical margin in 2 of these patients were noted to be positive; one patient had received neoadjuvant chemotherapy and one was in the surgery first group. The third local-regional recurrence occurred in a patient in the surgery first group who had negative margins. With a median follow-up of 4 years in the neoadjuvant group, no patients in this cohort with a negative margin developed recurrence.
Discussion
The role of neoadjuvant chemotherapy in patients with ILC is an area of controversy. Studies have shown that ILC is associated with lower pathologic complete response rates 11, 12. Patients with ILC have also been found to require more surgeries than patients with IDC in order to achieve negative margins 12, 13. Our study similarly shows that despite the lower response rates compared to IDC, about 20% of patients with ILC successfully undergo BCT after completing neoadjuvant chemotherapy. Given the unique histologic appearance of single file tumor infiltration seen in ILC compared to the broader invasive front seen with invasive ductal carcinoma, patchy response to chemotherapy may be more likely to compromise accurate margin assessment in ILC patients. Thus we wanted to determine whether margin width predicted for probability of residual disease and whether this was altered as a result of neoadjuvant chemotherapy. We found that most ILC patients who underwent BCT as the first operation after completing chemotherapy had positive margins and ultimately required mastectomy compared to patients that undergo surgery first. However the probability of finding residual disease as a function of margin width is not affected by the administration of neoadjuvant chemotherapy.
The role of breast conservation therapy for patients with ILC has been controversial, however a number of reports suggest that BCT can be safely performed in patients with early stage disease 5, 6, 8, 14. In particular, many studies have reported that women with ILC who undergo BCT have higher margin positivity rates after an initial attempt at breast conservation compared to patients with IDC. Although the definition of positive margin varies across studies, approximately 50% of women with ILC in these reports required reoperation for a close or positive margin 15, 16. We also found a 50% likelihood of an involved margin after BCT in the cohort of ILC patients who underwent surgery first. This number increases to almost 75% of the patients who received neoadjuvant chemotherapy. This likely reflects the radiologic and clinical limitations in accurately staging patients for local disease extent following chemotherapy combined with the lower rates overall of pathologic response seen in ILC patients.
A number of studies have evaluated the influence of margin width on the likelihood of finding additional tumor at the time of re-excision. Dillon et al found that patients with ILC with an initial margin of <5mm had a 60% likelihood of finding residual cancer. Moore and colleagues found that nearly half of the patients taken back for a second procedure had residual disease although the correlation with margin width was not detailed. In contrast we found that patients with margins >1mm have a low likelihood of residual invasive carcinoma or ductal carcinoma in situ and importantly, we saw no impact on the probability of residual disease based on the administration of neoadjuvant chemotherapy. Although the discrepancy between our study and others in the literature is not clear, one possibility might be the definition of residual disease, which in our study excluded LCIS. We opted to exclude LCIS given the preponderance of the data that shows presence of LCIS at margins of resection does not increase the probability of local recurrence 9, 10.
Although a few studies have reported a low risk for local recurrence after incomplete tumor excision in ILC 17, in general the goal of surgical excision remains negative margins to minimize the residual tumor burden and hence local recurrence risk 18. Local recurrence rates after BCT for early stage ILC have been reported by a number of institutions. Hussein and colleagues reported a 43% rate of LR for women treated with breast conservation therapy 19. However, when further analyzed all the recurrences were among the women who declined radiation therapy after segmental mastectomy. In contrast, a review of ILC cases through the Eindhoven Cancer Registry in the Netherlands, showed a 5 year risk of LR of 3.5% and this was not affected by a positive surgical margin. Our own previous experience suggests a similar low risk of LR events in women with Stage I and II ILC who undergo BCT with a margin of >1mm 4, 8. Our current report similarly shows a low rate of LR among all women with ILC who undergo neoadjuvant chemotherapy. There are a number of limitations to our current analysis. First, our cohort of women with BCT following neoadjuvant chemotherapy is small and second, our median followup for the neoadjuvant chemotherapy is 4 years and thus a substantial number of late recurrences cannot be ruled out. In order to overcome these limitations, we analyzed our cohort to look at probability of residual disease as a function of margin width and used residual disease as a surrogate for long-term risk of LR. In this analysis, we found that women in both the surgery first and the neoadjuvant chemotherapy groups had similar rates of residual disease when stratified by an initial margin of 1mm. Given our previous experience that a >1mm margin in ILC patients without neoadjuvant chemotherapy translates into low rates of local recurrence at long-term follow-up, we thus presume that similarly low rates of residual disease in women after neoadjuvant chemotherapy who have a >1mm margin will translate into good long-term local control.
In conclusion, patients with locally advanced ILC who undergo BCT following neoadjuvant chemotherapy have high probability of margin positive resection and requirement for a second operation. A >1mm margin appears to be acceptable for minimizing residual disease and local recurrence risks in these patients.
Synopsis.
Patients with invasive lobular carcinoma who undergo an initial attempt at breast conservation surgery following neoadjuvant chemotherapy have high likelihood of margin involvement and need for second surgery. However, a final margin width of >1mm appears to be sufficient for good long-term local control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tavassoli FADP. Pathology & Genetics: Tumours of the Breast and Female Genital Organs. IARC Press; 2003. World Health Organization Classification of Tumours; pp. 23–26. [Google Scholar]
- 2.Feig BWBD, Fuhrman GM. The MD Anderson surgical oncology handbook. 4. 2006. pp. 23–59. [Google Scholar]
- 3.Singletary SE, Patel-Parekh L, Bland KI. Treatment trends in early-stage invasive lobular carcinoma: a report from the National Cancer Data Base. Ann Surg. 2005;242(2):281–289. doi: 10.1097/01.sla.0000171306.74366.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvet M, Ollila DW, Hunt KK, et al. Role of conservation therapy for invasive lobular carcinoma of the breast. Ann Surg Oncol. 1997;4(8):650–654. doi: 10.1007/BF02303750. [DOI] [PubMed] [Google Scholar]
- 5.Salvadori B, Biganzoli E, Veronesi P, et al. Conservative surgery for infiltrating lobular breast carcinoma. Br J Surg. 1997;84(1):106–109. [PubMed] [Google Scholar]
- 6.Peiro G, Bornstein BA, Connolly JL, et al. The influence of infiltrating lobular carcinoma on the outcome of patients treated with breast-conserving surgery and radiation therapy. Breast Cancer Res Treat. 2000;59(1):49–54. doi: 10.1023/a:1006384407690. [DOI] [PubMed] [Google Scholar]
- 7.Holland PA, Shah A, Howell A, et al. Lobular carcinoma of the breast can be managed by breast-conserving therapy. Br J Surg. 1995;82(10):1364–1366. doi: 10.1002/bjs.1800821023. [DOI] [PubMed] [Google Scholar]
- 8.Vo TN, Meric-Bernstam F, Yi M, et al. Outcomes of breast-conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg. 2006;192(4):552–555. doi: 10.1016/j.amjsurg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Stolier AJ, Barre G, Bolton JS, et al. Breast conservation therapy for invasive lobular carcinoma: the impact of lobular carcinoma in situ in the surgical specimen on local recurrence and axillary node status. Am Surg. 2004;70(9):818–821. [PubMed] [Google Scholar]
- 10.Ciocca RM, Li T, Freedman GM, Morrow M. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol. 2008;15(8):2263–2271. doi: 10.1245/s10434-008-9960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 12.Cocquyt VF, Blondeel PN, Depypere HT, et al. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29(4):361–367. doi: 10.1053/ejso.2002.1404. [DOI] [PubMed] [Google Scholar]
- 13.Tubiana-Hulin M, Stevens D, Lasry S, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17(8):1228–1233. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- 14.Mai KT, Yazdi HM, Isotalo PA. Resection margin status in lumpectomy specimens of infiltrating lobular carcinoma. Breast Cancer Res Treat. 2000;60(1):29–33. doi: 10.1023/a:1006359308505. [DOI] [PubMed] [Google Scholar]
- 15.Moore MM, Borossa G, Imbrie JZ, et al. Association of infiltrating lobular carcinoma with positive surgical margins after breast-conservation therapy. Ann Surg. 2000;231(6):877–882. doi: 10.1097/00000658-200006000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon MF, Hill AD, Fleming FJ, et al. Identifying patients at risk of compromised margins following breast conservation for lobular carcinoma. Am J Surg. 2006;191(2):201–205. doi: 10.1016/j.amjsurg.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 17.van den Broek N, van der Sangen MJ, van de Poll-Franse LV, et al. Margin status and the risk of local recurrence after breast-conserving treatment of lobular breast cancer. Breast Cancer Res Treat. 2007;105(1):63–68. doi: 10.1007/s10549-006-9431-5. [DOI] [PubMed] [Google Scholar]
- 18.Schnitt SJ, Connolly JL, Recht A, et al. Influence of infiltrating lobular histology on local tumor control in breast cancer patients treated with conservative surgery and radiotherapy. Cancer. 1989;64(2):448–454. doi: 10.1002/1097-0142(19890715)64:2<448::aid-cncr2820640218>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Hussien M, Lioe TF, Finnegan J, Spence RA. Surgical treatment for invasive lobular carcinoma of the breast. Breast. 2003;12(1):23–35. doi: 10.1016/s0960-9776(02)00182-0. [DOI] [PubMed] [Google Scholar]


