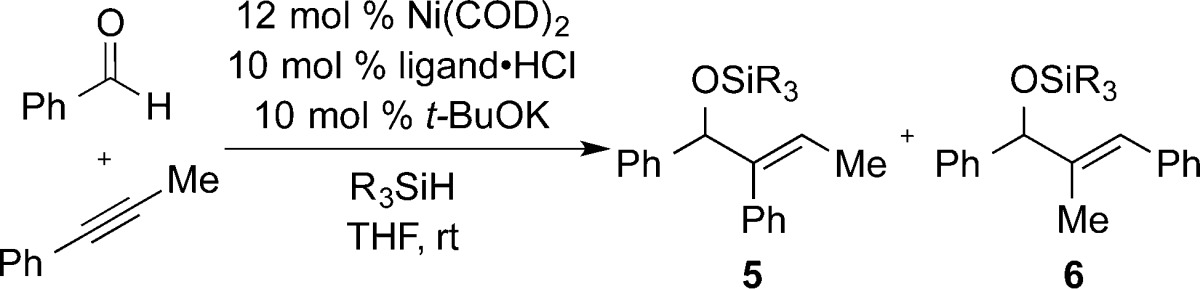
Table 1. Ligand and Silane Structural Effectsa.
| entry | ligand | R3SiH | 5:6 (% yield) |
|---|---|---|---|
| 1 | IMes | Et3SiH | <2:98 (84) |
| 2 | IMes | (i-Pr)3SiH | <2:98 (83) |
| 3 | SIMes | Et3SiH | 4:96b |
| 4 | SIMes | (i-Pr)3SiH | 4:96b |
| 5 | IPr | Et3SiH | 44:56b |
| 6 | IPr | (i-Pr)3SiH | 56:44b |
| 7 | SIPr | Et3SiH | 58:42 (65) |
| 8 | SIPr | t-BuMe2SiH | 60:40 (83) |
| 9 | SIPr | t-BuPh2SiH | 77:23 (86) |
| 10 | SIPr | (i-Pr)3SiH | 83:17 (89) |
| 11 | SIPr | (t-Bu)2MeSiH | >98:2 (61) |
Ni(COD)2 (0.06 mmol), ligand·HCl (0.05 mmol), and t-BuOK (0.05 mmol) were stirred with 2 mL of THF. Benzaldehyde (0.5 mmol), phenylpropyne (0.5 mmol), and silane (1.0 mmol) were combined, diluted to a total volume of 2 mL, and added to the reaction mixture via syringe drive over 1 h at rt. IMes·Cl = 1,3-bis(mesityl)imidazolium chloride; SIMes·HCl = 1,3-bis(mesityl)-4,5-dihydroimidazolium chloride; IPr·HCl = 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride; SIPr·HCl = 1,3-bis(2,6-diisopropyl-phenyl)-4,5-dihydroimidazolium chloride.
Isolated yield not determined.