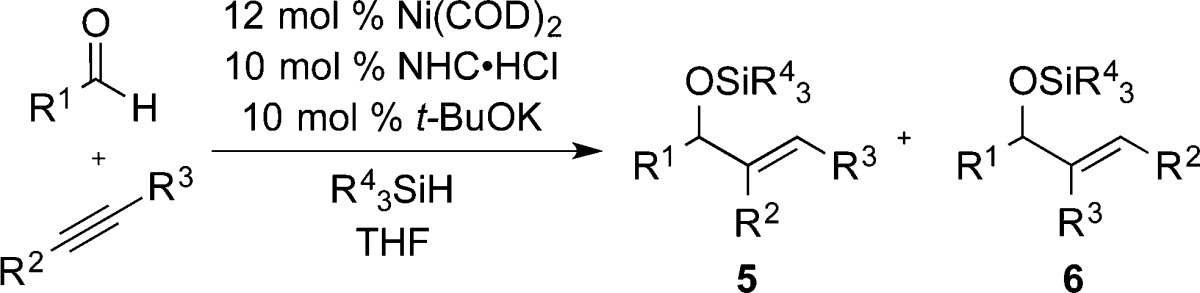
Table 4. Reaction Scope with Optimized Protocol.
| entry | R1 | R2 | R3 | methoda | 5:6 (% yield) |
|---|---|---|---|---|---|
| 1 | Ph | Ph | Me | Ab | >98:2 (82) |
| 2 | 4-FC6H4 | Ph | Me | A | 93:7 (85) |
| 3 | n-Hept | Ph | Me | A | >98:2 (77) |
| 4 | c-Hex | Ph | Me | A | >98:2 (90) |
| 5 | Ph | i-Bu | Et | A | 94:6 (86) |
| 6 | n-Hept | i-Bu | Et | A | 93:7 (66) |
| 7 | Ph | n-Pr | Et | A | 68:32 (56) |
| 8 | 2-furyl | n-Pr | Me | A | 93:7 (76) |
| 9 | Ph | i-Pr | Me | A | >98:2 (78) |
| 10 | c-Hex | i-Pr | Me | A | >98:2 (75) |
| 11 | Ph | i-Pr | H | B | >98:2 (61) |
| 12 | Ph | n-Hex | H | B | 95:5 (69) |
Method A: i-Pr3SiH as reductant, reaction conducted at 0.125 M in the aldehyde and alkyne at 50 °C. Method B: t-Bu2MeSiH as reductant, reaction conducted at 0.0125 M in the aldehyde and alkyne with 22 mol % Ni(COD)2 at rt.
Conducted at 0.0125 M in the aldehyde and alkyne.