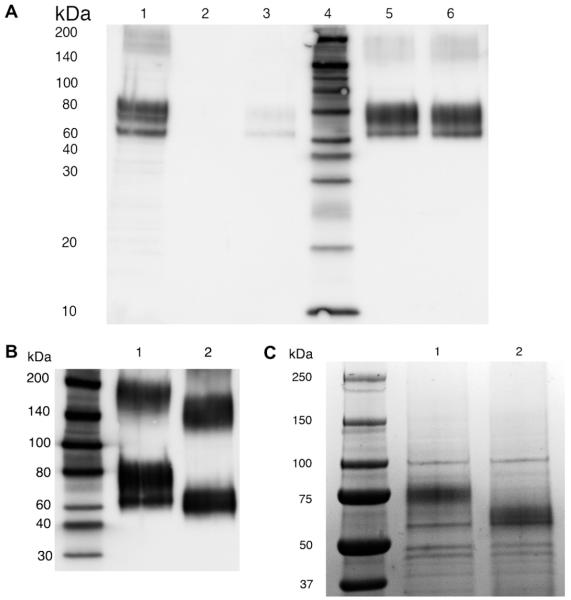
Fig. 4.
IMAC purification of 6×HIS hEAAT2. (A) Western blot to detect recombinant 6×HIS hEAAT2. Lane 1: resuspend pellet from second high speed spin prior to application to IMAC column. Lane 2: 1:200 dilution of 1st column eluent (50 mM imidazole) from the Ni2+-chelating column. Lane 3: 1:200 dilution of 2nd column eluent (100 mM imidazole) from the Ni2+-chelating column. Lane 4: biotin ladder standard. Lane 5: 1:200 dilution of 3rd column eluent (150 mM imidazole) from the Ni2+-chelating column. Lane 6: 1:200 dilution of 4th column eluent (200 mM imidazole) from the Ni2+-chelating column. (B) Western blot probed with anti-GLT-1 antibody to show the glycosylation of 6×HIS hEAAT2. Lane 1: 1:200 dilution of 200 mM imidazole fraction eluted from the Ni2+-chelating column. Lane 2: deglycosylated 6×HIS hEAAT2 treated by PNGase F. (C) Coomassie blue stained SDS–PAGE gel (20 μl of concentrated samples from 200 μl 150 mM imidazole fraction eluted from the Ni2+-chelating column). Lane 1: control. Lane 2: PNGase F deglycosylation.