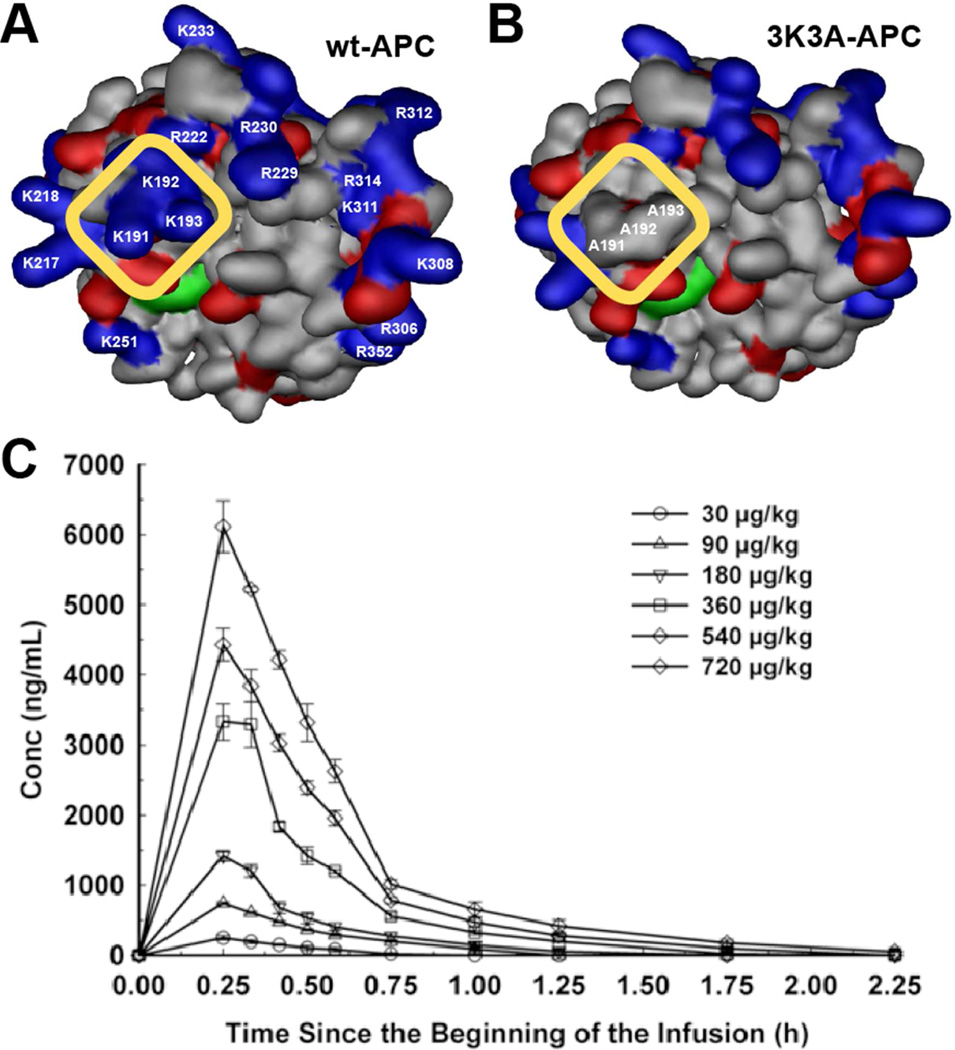
Figure 6. Dosing of cytoprotective-selective 3K3A-APC in healthy adult volunteers.
The surface contours for the APC protease domain showing positive (blue) and negative (red) side-chains for wt-APC (A) and 3K3A-APC (B). The yellow rectangle highlights the comparison of the protease domain for 3K3A-APC (containing 3 Ala substitutions at Lys191, Lys192, and Lys193) with wt-APC to indicate the loss of three positively charged surface residues from an exosite that is required for normal recognition of factor Va. Loss of this factor Va-binding site results in reduced anticoagulant activity (~5% of wt-APC) but not any loss of cytoprotective actions, hence the designation “cytoprotective-selective” for the 3K3A-APC variant. (C) As the result of its reduced anticoagulant activity, 3K3A-APC can reach high plasma concentrations without adverse effects in healthy human subjects after a brief i.v. infusion of a single dose (data in panel C) or after five repeated high doses every 12 h (data not shown). Panel C is from Lyden et al. Curr Pharm Design 2013 (89).