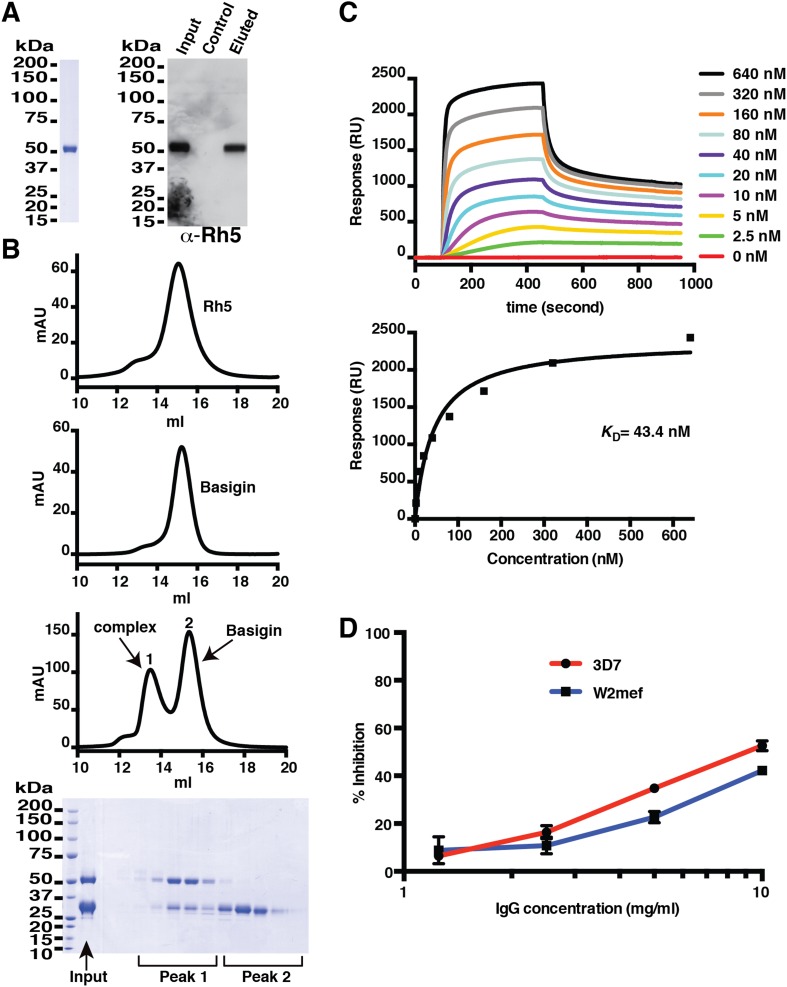
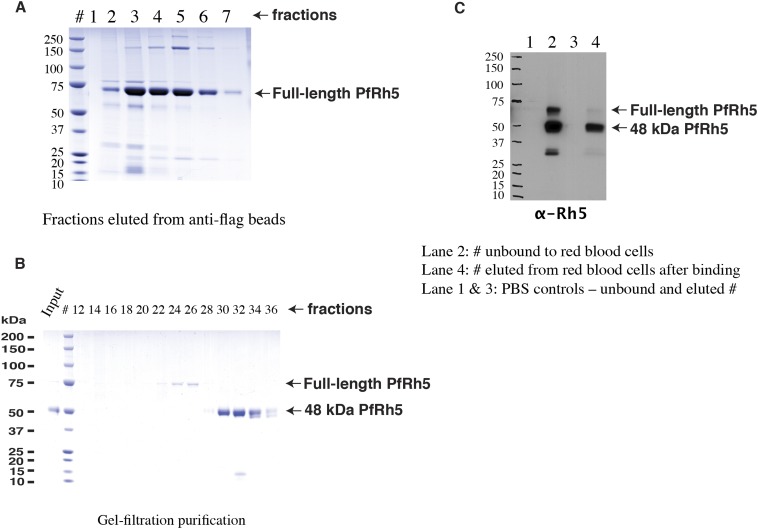
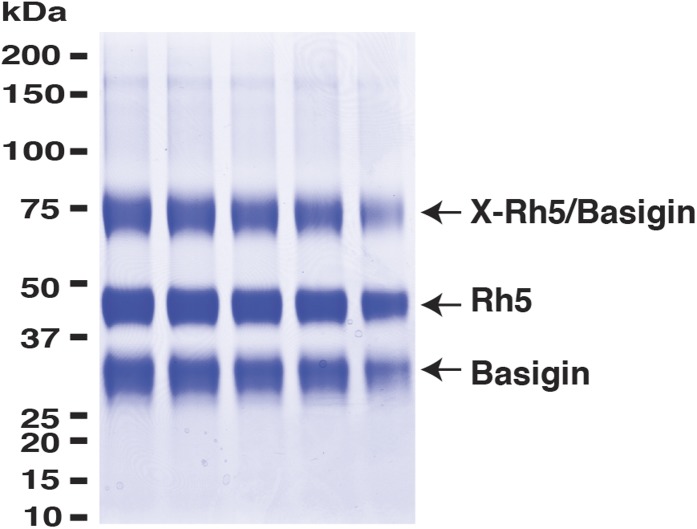
Figure 1. Production of functional recombinant PfRh5.
(A) Purified recombinant PfRh5 was analysed by SDS-PAGE analyses and by erythrocyte binding assays. (B) Formation of the PfRh5–basigin complex was monitored by size-exclusion chromatography. The chromatographic profiles are shown for PfRh5 (panel 1), basigin (panel 2), and the PfRh5-basigin complex (panel 3). The fractions eluted from the column in panel 3 were analysed by SDS-PAGE. (C) The binding affinity of the recombinant PfRh5 to human basigin was measured by SPR on Biacore 3000 with the basigin coupled to a sensor chip. (D) In vitro growth inhibition assays were performed to assess the abilities of the polyclonal antibodies to the recombinant PfRh5 in blocking P. falciparum parasite invasion into erythrocytes.