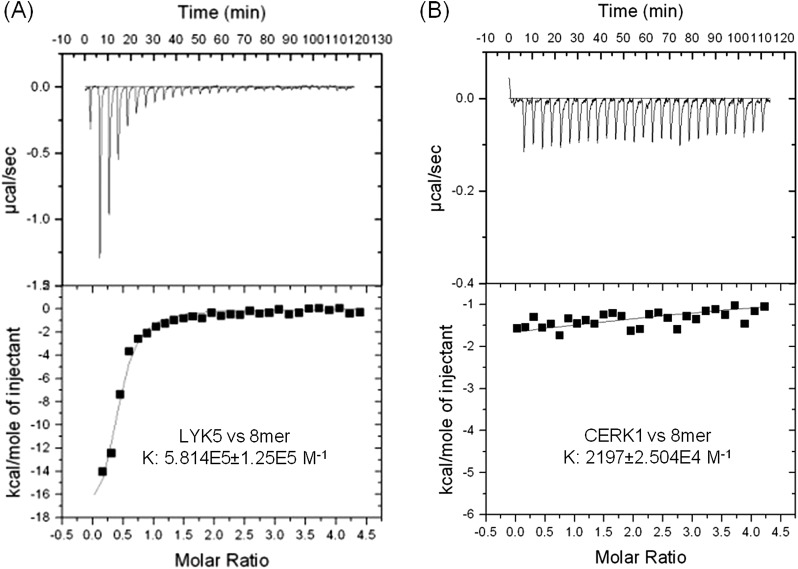
Figure 3. AtLYK5 shows stronger chitin binding affinity than AtCERK1.
The binding affinity of AtLYK5 (A) and AtCERK1 (B) to chitooctaose (GlcNAc)8 was measured using isothermal titration calorimetry (ITC). Proteins were purified from E. coli. Upper panels and lower panels indicate raw data and integrated heat values, respectively.