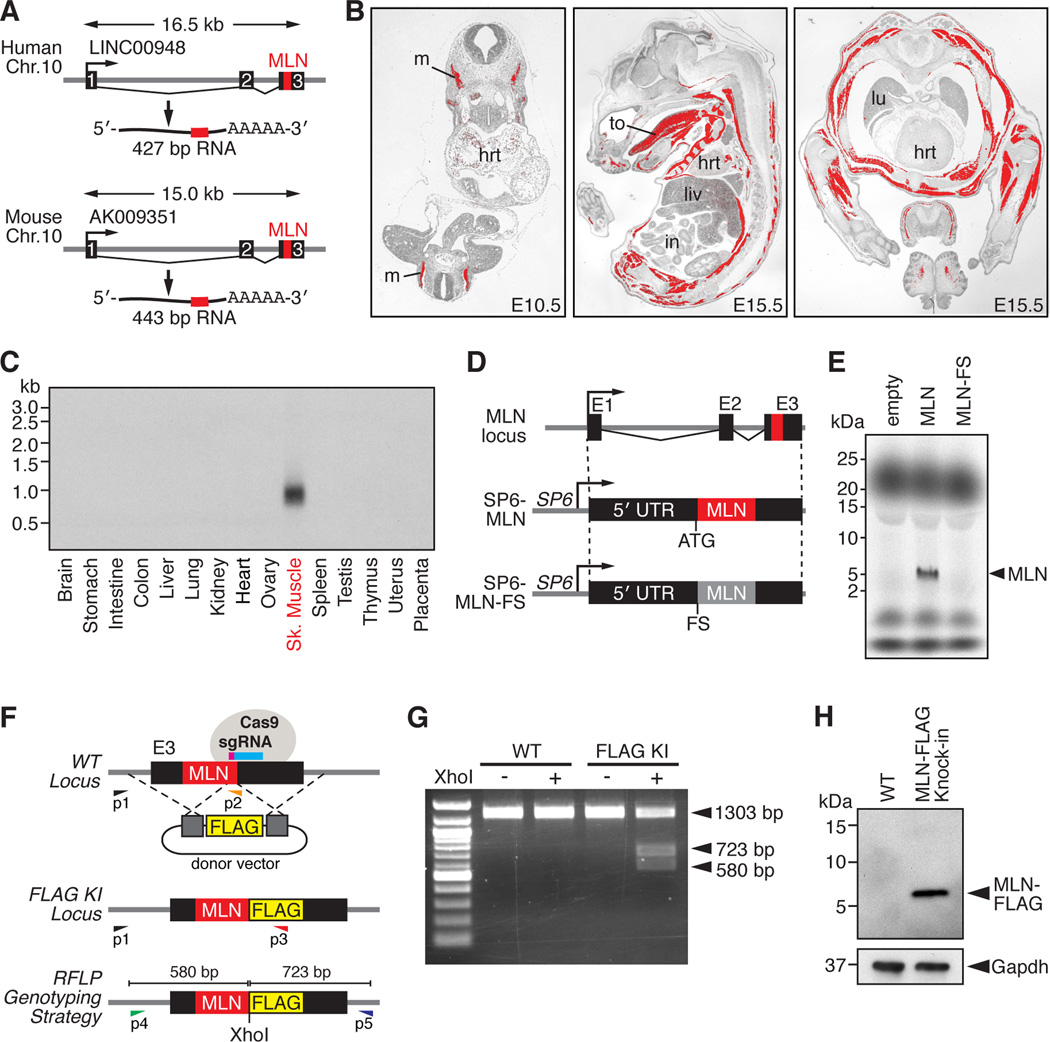
Figure 1. Discovery of a Skeletal Muscle-specific Micropeptide.
(A) A short ORF encoding a conserved micropeptide, that we named myoregulin (MLN), is contained within exon 3 of an annotated lncRNA in human and mouse genomes. The position of the MLN ORF is indicated in red.
(B) In situ hybridization showing skeletal muscle-specific expression of MLN at the indicated embryonic time-points. (hrt, heart; in, intestine; liv, liver; lu, lung; m, myotome; to, tongue)
(C) Northern blot of RNA isolated from adult mouse tissues using a probe specific to the full-length MLN transcript shows skeletal muscle-specific expression.
(D) Diagram of the constructs used for in vitro translation of the MLN micropeptide. The full-length MLN RNA transcript was subcloned into the CS2 vector containing the SP6 phage RNA polymerase promoter (SP6-MLN). A frameshift mutation was introduced immediately after the endogenous ATG to disrupt the MLN ORF (SP6-MLN-FS).
(E) Coupled in vitro transcription and translation reactions of the SP6-MLN vector using radiolabeled 35S-methionine produced a ∼5 kDa micropeptide, visualized by Tricine SDS-PAGE. The frame-shift mutation in the MLN ORF (SP6-MLN-FS) abolished any detectable expression.
(F) Targeting strategy using CRISPR/Cas9-mediated homologous recombination to knock-in a FLAG epitope tag into the MLN locus in C2C12 cells. PCR-based genotyping using primers (P1-P3) or RFLP analysis of PCR products generated using primers (P4 and P5) were used to verify correct targeting.
(G) RFLP analysis of WT C2C12 and heterozygous C2C12 myoblasts for the MLN-FLAG knock-in allele.
(H) Western blot analysis showing endogenous expression of the MLN-FLAG fusion peptide in differentiated C2C12 myotubes, detected with an anti-FLAG antibody.
See also Figure S1.