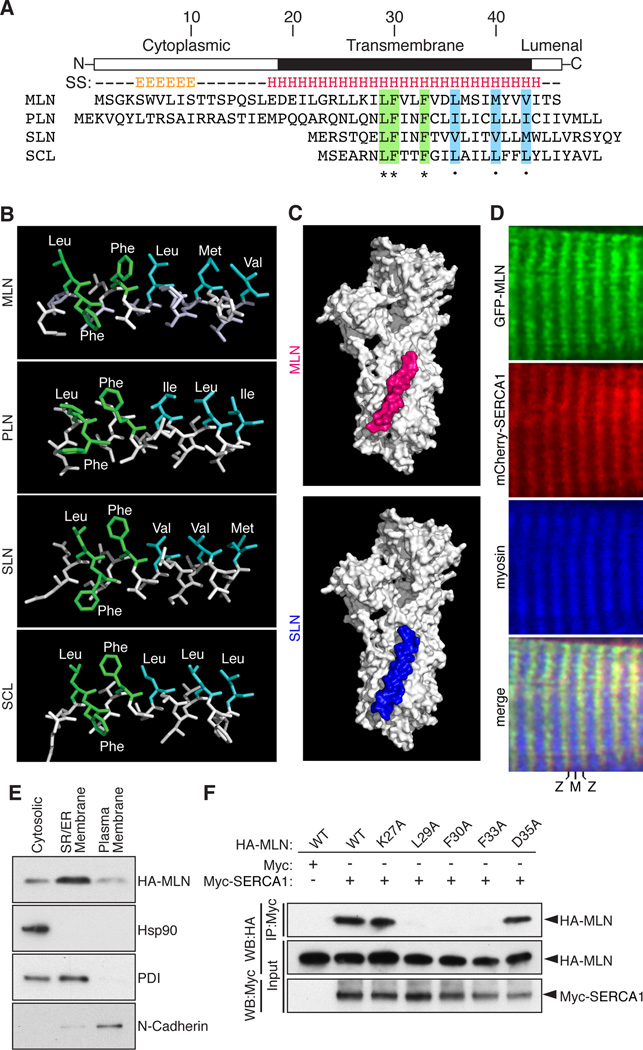
Figure 2. MLN Forms a Transmembrane Alpha Helix that Interacts with SERCA.
(A) The secondary structure (SS) of MLN is predicted to contain an N-terminal beta sheet and C-terminal transmembrane alpha helix (E, beta sheet; H, alpha helix). Alignment of mouse MLN, PLN, SLN and the invertebrate ortholog SCL identified identical (green) and similar (blue) residues conserved in all four micropeptides.
(B) The structure of the transmembrane helices of MLN, SLN, PLN and SCL was modeled using I-TASSER and showed a common spatial orientation of conserved residues.
(C) Automated protein docking using ClusPro predicted that MLN occupies the same groove in SERCA1 that is recognized by SLN.
(D) Expression of GFP-MLN and mCherry-SERCA1 fusion proteins in mature skeletal muscle fibers, showing co-localization in the SR, imaged using two-photon laser scanning confocal microscopy. (M, M-line; Z, Z-line)
(E) Retroviral expression of an N-terminal HA-tagged MLN fusion peptide (HA-MLN) in C2C12 myoblasts was enriched in the subcellular fraction containing SR/ER membrane proteins. Enrichment for cytosolic, SR/ER membrane and plasma membrane proteins was verified by Western blot analysis for Hsp90, PDI and N-Cadherin, respectively.
(F) Co-immunoprecipitation (CoIP) experiments with HA-tagged MLN alanine mutants and a Myc-tagged SERCA1 construct transfected into COS7 cells identified residues important for interaction of MLN with SERCA1. Interaction of MLN with SERCA1 was abolished by mutation of residues shared with PLN, SLN and SCL (L29A, F30A and F33A) but not by the charged residues K27A or D35A. Western blot performed with anti-HA (WB-HA) or anti-Myc (WB-Myc). See also Figure S2.