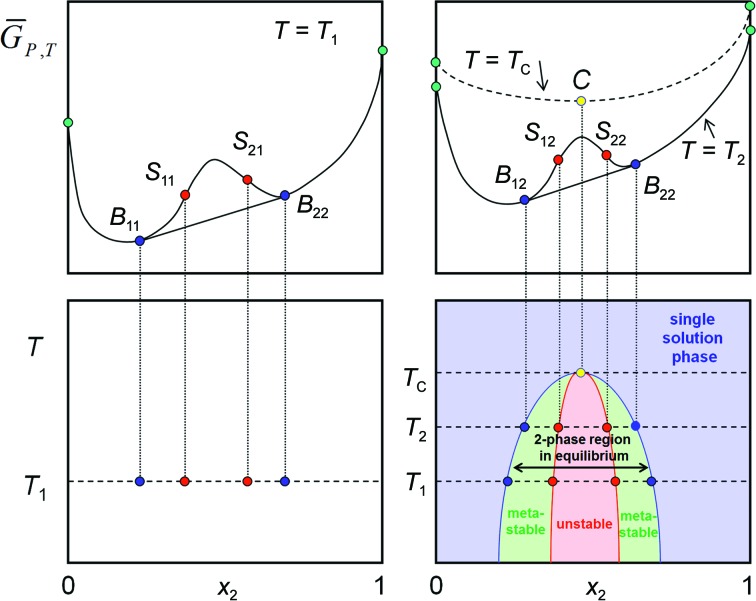
Figure 6.
Constructing the T/x diagram. The complete T/x diagram of a simple two-component system with a miscibility gap is obtained by projecting binodal equilibrium points and the spinodal decomposition points from each  isotherm onto corresponding isotherms of a temperature–composition (T/x) diagram. The blue solubility line delineates the phase boundary of the single solution phase region. The left boundary phases are rich in component 2 and the right boundary phases are rich in component 1. In thermodynamic equilibrium, the region between the solubility lines is a two-phase mixture of the two boundary phases in ratios according to the lever rule. A metastable phase region exists between the binodal coexistence line (or solubility line; blue) and the spinodal decomposition line (red). In the red instability region, instantaneous spinodal decomposition into the corresponding boundary phases must take place.
isotherm onto corresponding isotherms of a temperature–composition (T/x) diagram. The blue solubility line delineates the phase boundary of the single solution phase region. The left boundary phases are rich in component 2 and the right boundary phases are rich in component 1. In thermodynamic equilibrium, the region between the solubility lines is a two-phase mixture of the two boundary phases in ratios according to the lever rule. A metastable phase region exists between the binodal coexistence line (or solubility line; blue) and the spinodal decomposition line (red). In the red instability region, instantaneous spinodal decomposition into the corresponding boundary phases must take place.