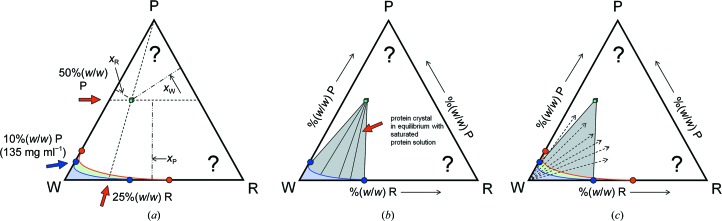
Figure 9.
Location of crystal phase fields. (a) Assuming a crystal with 50% solvent content at 25% PEG, the crystal contains 50% protein, 12.5% PEG and 37.5% water. The phase point can be located in the phase diagram as follows. All concentrations with a fixed absolute amount of a component P are on lines parallel to the opposite side (WR). Lines indicating a fixed ratio of W:R lead towards the remaining component (P). The intersection of these two lines is the sought-after phase point (or field, if any homogeneity range exists) of the crystal. The sum of the three fractions xi is constrained to one. (b) In equilibrium, i.e. the end point of the crystallization experiment, the crystal will coexist in equilibrium with saturated protein solution with concentration [W, P, R] as defined by the corresponding coexistence line originating from the solubility line. (c) In this panel, the equilibrium information is combined with the existence region of the metastable supersaturated phase (green). If only a single component such as water is removed in a vapour-diffusion experiment, the initial ratio of P:R remains constant and therefore the path which the experiment takes leads away from the water corner. The arrows indicate only the direction of the pathways in a vapour-diffusion experiment (described in detail in Fig. 10 ▶). Note that the actual experimental path must invariably end at the red spinodal decomposition line. Outside of the grey two-phase field indicating saturated solution–protein crystal equilibria, spontaneously formed mixtures of the corresponding boundary phases exist. We can see that the actual experimental workspace will be very compressed towards the water corner; note the unusually protein high solubility in this particular example.