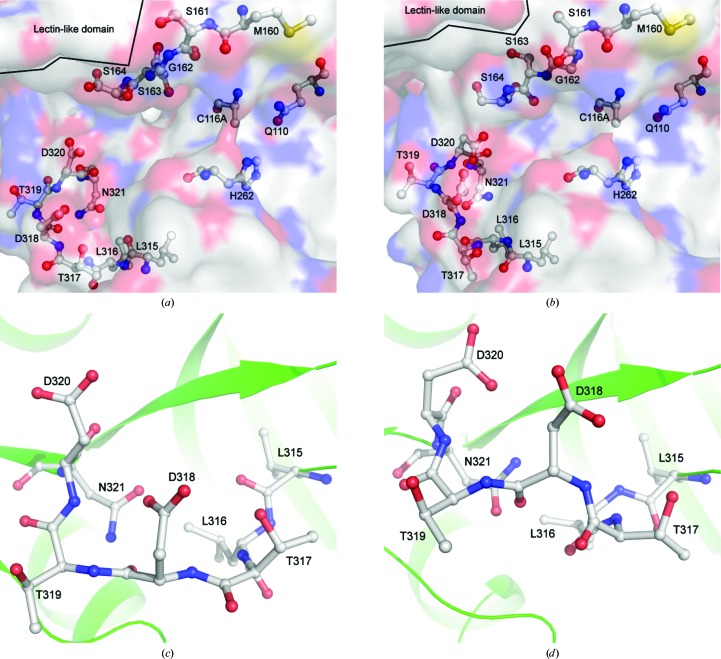
Figure 4.
Conformational changes in the active-site groove upon propeptide cleavage. (a) The active-site conformation with the propeptide bound. The catalytic dyad (C116A and His262) and the oxyanion hole-forming glutamine (Gln110) are shown along with the loops formed by Met160–Ser164 and Leu315–Asp320 at the top and the bottom left, respectively. Ser161 and Ser164 exhibit multiple conformations. (b) The active site after propeptide cleavage. Significant rotations can be observed for Gly162 and Ser163, resulting in a change in the shape of the S1 pocket. This is owing to the disruption of a hydrogen-bond network with the propeptide and lectin-like domain, the latter of which is highlighted and can be seen to move away from the active-site groove. The S2 loop, and particularly Asp318, can be seen to move closer to the catalytic residues upon propeptide cleavage, occluding much of the previously identified P2 pocket. This does, however, result in a negatively charged surface formed by Asp318 and Asp320, which may be better suited to binding the P2 lysine of SlpA. (c) Close-up of the S2 loop before propeptide cleavage. (d) Close-up of the S2 loop after propeptide cleavage. Significant movement can be seen, particularly for Asp318.