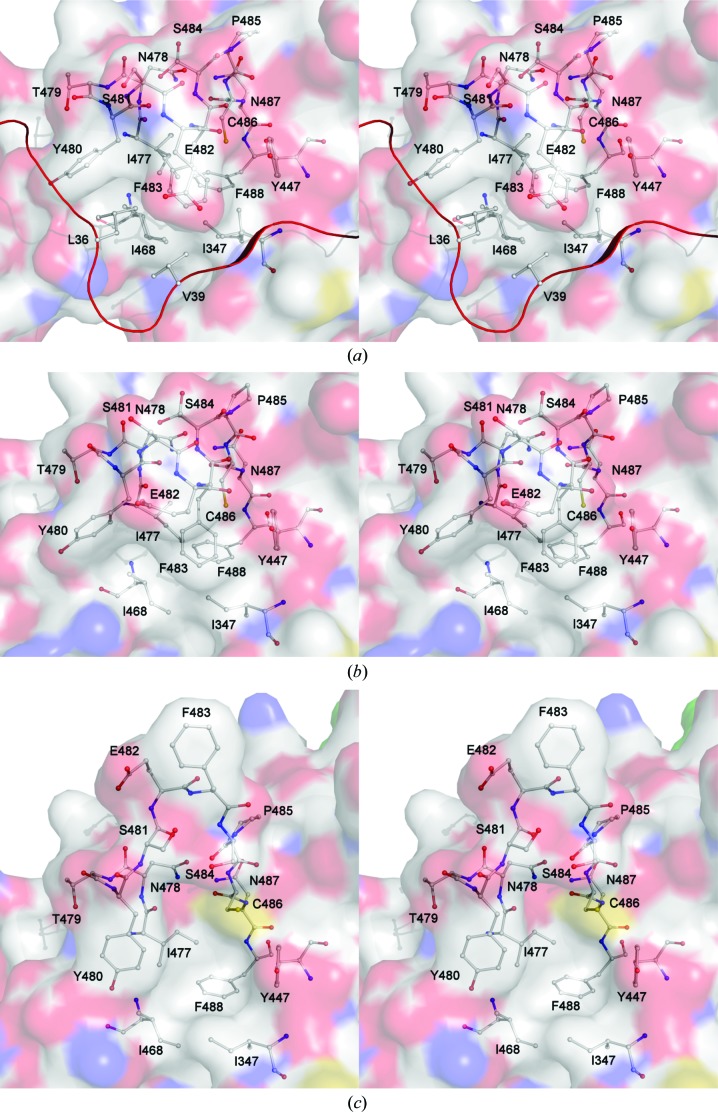
Figure 5.
Cross-eyed stereoview of the conformational change of the hydrophobic pocket on the surface of the lectin-like domain upon propeptide cleavage. Residues forming the hydrophobic pocket and the loop formed by Thr479–Cys486 are shown. (a) The hydrophobic pocket with the propeptide bound; a portion of the propeptide is shown as a ribbon, with Leu36 and Val39 shown as sticks. Ile347, Ile468, Ile477 and Ser484 exhibit multiple conformations. (b) The hydrophobic pocket in structure 1. The conformation is similar to that with the propeptide, but the pocket is slightly more open. Ser484 exhibits multiple conformations. (c) The hydrophobic pocket in structure 2. The stabilizing effect of the propeptide is lost, allowing increased flexibility. This results in the pocket having greater accessibility, including the exposure of previously occluded residues.