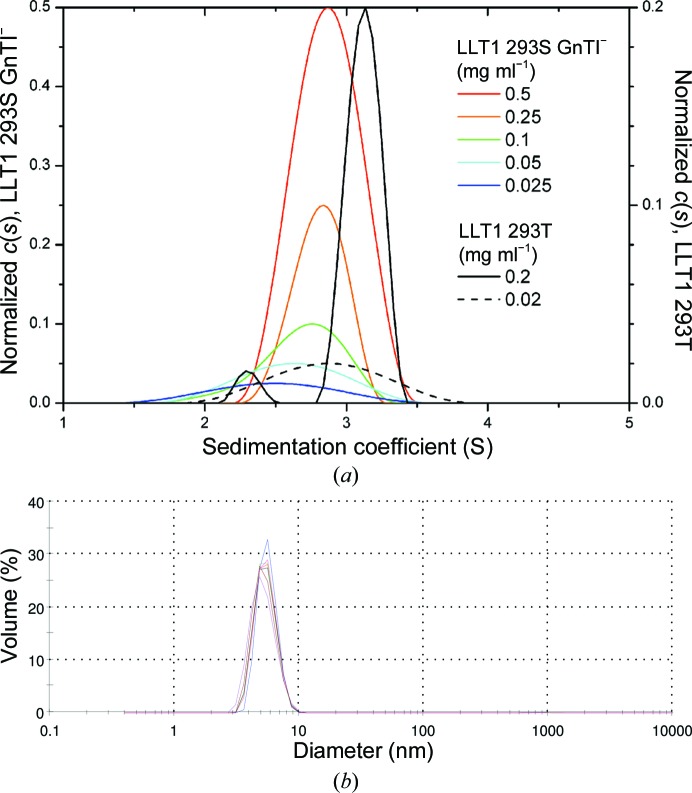
Figure 7.
(a) Sedimentation analysis of LLT1 oligomerization. Glycosylated LLT1 with homogeneous GlcNAc2Man5 N-glycosylation produced in the HEK293S GnTI− cell line was characterized by a sedimentation-velocity experiment in an analytical ultracentrifuge at five different concentrations (coloured lines). The continuous size distribution of the sedimenting species is shown, which was normalized with respect to the difference in loading concentration. A broad size distribution that is shifted towards lower values with lower protein concentration points to monomer–dimer equilibrium; for comparison, data for LLT1 produced in the HEK293T cell line with wild-type complex N-glycosylation are shown (black and dashed lines), with separated signals for dimeric and monomeric protein at higher protein concentration. (b) Size distribution of LLT1 measured by dynamic light scattering and scaled by volume. Seven measurements (distributions differentiated by colour) were performed with similar results, three of them at 291 K, two at 298 K and two at 303 K.