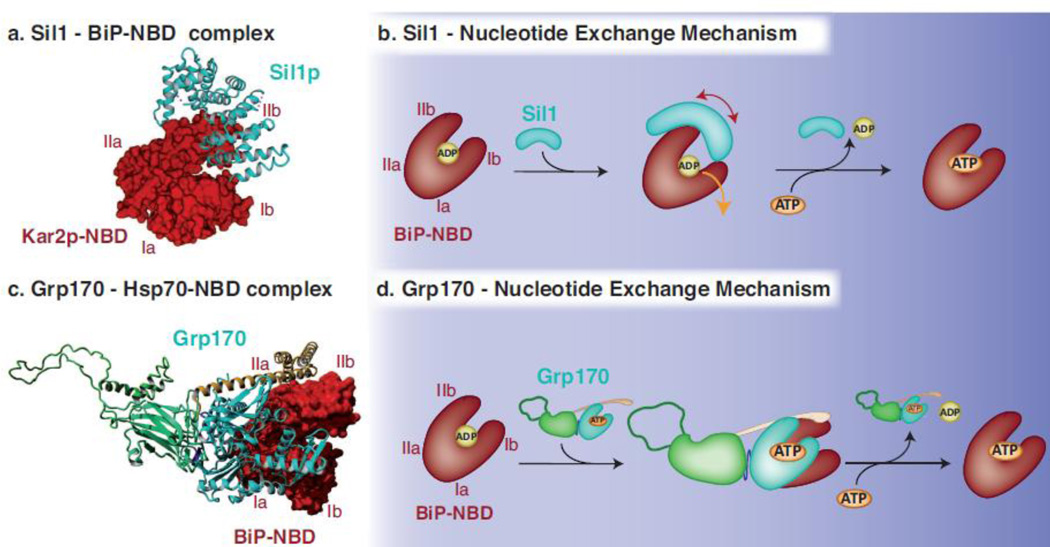
Fig. 4. Mechanisms used by Sil1 and Grp170 to regulate nucleotide exchange for BiP.
(a) The crystal structure obtained for yeast Sil1 bound to the NBD of BiP (PDB: 3QML)88 depicts how the single domain NEF Sil1, consisting of four Armadillo-like repeats (cyan, shown in ribbon), wraps around lobe IIb of BiP’s NBD (red, shown in surface representation) and displaces lobe Ib during the nucleotide exchange reaction. (b) Upon the binding of Sil1 to the ADP-bound form of BiP, the NBD cleft of BiP is opened by tilting lobe IIb and to a lesser extent lobe Ib outwards and thus destabilizing the domain and releasing ADP. ATP can subsequently bind to the NBD and BiP can re-enter its ATPase cycle. (c) The crystal structure of yeast Sse1p bound to the NBD of human Hsp70 (PDB: 3D2F)112 was used to model Grp170 (NBD in cyan, β-sheet and unstructured loop in green and α-helical domain in yellow, shown in ribbon) using Yasara Structure (www.yasara.org). Grp170 is shown bound to the human BiP NBD (3LDL)30 (red, shown in surface representation). Complex formation occurs via multiple contacts between the respective NBDs, and in addition, the C-terminal α-helical domain of Grp170 reaches out to embrace the Hsp70 NBD. (d) For the nucleotide exchange reaction to occur, Grp170 apparently binds to the ADP-bound form of BiP and destabilizes the structure of BiP´s NBD resulting in the release of ADP. Once ATP is bound to BiP, the Grp170-BiP complex would dissociate and BiP can re-enter its ATPase cycle, although key steps in the NEF activity for large Hsp70 proteins remain to be elucidated.