Abstract
One of the most important proarrhythmic complications after left atrial (LA) ablation is regular atrial tachycardia (AT) or flutter. Those tachycardias that occur after atrial fibrillation (AF) ablation can cause even more severe symptoms than those from the original arrhythmia prior to the index ablation procedure since they are often incessant and associated with rapid ventricular response. Depending on the method and extent of LA ablation and on the electrophysiological properties of underlying LA substrate, the reported incidence of late ATs is variable. To establish the exact mechanism of these tachycardias can be difficult and controversial but correlates with the ablation technique and in the vast majority of cases the mechanism is reentry related to gaps in prior ablation lines. When tachycardias occur, conservative therapy usually is not effective, radiofrequency ablation procedure is mostly successful, but can be challenging, and requires a complex approach.
Keywords: Atrial fibrillation ablation, pulmonary vein isolation, atrial tachycardia, peri-mitral flutter.
INTRODUCTION
Over the last decades, pulmonary vein isolation (PVI) has become the mainstay of ablation treatment of paroxysmal atrial fibrillation (AF), however the success rate of PVI alone remained suboptimal in the persistent AF population. This observation has led to the development of adjunctive ablation strategies targeting the left atrial (LA) substrate itself, creating linear radiofrequency (RF) lesions or eliminating complex fractionated left atrial electrograms (CFAE).
Regardless of the ablation techniques used, RF ablation of AF may result in regular atrial tachycardias (ATs) or flutter, which is one of the most important proarrhythmic complications after LA ablation.
Atrial tachycardias that occur after AF ablation can cause even more severe symptoms than those from the original arrhythmia prior to the index ablation procedure since they are often incessant and associated with rapid ventricular response, which is difficult to control using antiarrhythmic medications.
INCIDENCE
Depending on the method and extent of left atrial ablation and on the electrophysiological properties of underlying LA substrate, the reported incidence of late ATs is variable. The less is the harm caused in the LA, the lower is the incidence of late AT. Consequently using the “initial” approach of segmental electrical PVI, which is limited to the ostia of pulmonary veins (PV) the occurrence was found to be relatively low, between 1% and 2.9% [1-3]. Oral et al. [4] demonstrated in a prospective randomized study that in patients with symptomatic paroxysmal atrial fibrillation LA ablation to encircle the pulmonary veins is more effective than segmental ostial catheter ablation in terms of freedom from symptoms. Also Gaita et al. [5] concluded that in persistent atrial fibrillation, pulmonary vein isolation plus left atrial linear lesion is superior to pulmonary vein isolation alone in maintaining sinus rhythm. However, when PV ablation was achieved by placing circular lesions around the veins in the LA antrum, without targeting complete isolation of pulmonary veins, and creating additional lines in LA (e.g. mitral isthmus, and/or roof, or posterior lines) the incidence of ATs dramatically increased to even 10 fold higher, ranging from 10% to 24 %, [6, 7].
Karch et al. [8] conducted a randomized study to compare segmental pulmonary vein ablation approach, which electrically isolates the pulmonary veins to circumferential pulmonary vein ablation approach plus creating linear lesions in the left atrial myocardium, without isolation of veins or electrical completeness of lines. Atypical flutter was observed in 18% of patients after circumferential ablation versus 2% (p<0.001) after complete isolation of veins, which drew the attention to the importance of incomplete lines in arrhythmogenesis. In accordance with this observation, Sawhney et al. [9] randomized a series of patients with structurally normal hearts and paroxysmal AF to PVI with electrical isolation versus circular PV ablation plus additional lines with confirmation of completeness of circular and linear lines. After isolation of PVs without additional lines there were no cases with left ATs, but 24% had AT in the other group, despite having originally complete lines, without difference in AF recurrence rate. The authors concluded that in patients without structural heart disease linear ablation should be avoided as an initial ablation approach.
Nademanee [10] was the first, who reported a different approach focusing on complex fractionated atrial electrograms (CFAE) as a target of ablation procedure mainly in persistent AF population. The incidence of LA AT was 8 %. Later, Estner and co-authors demonstrated in their randomized study [11] that comparing PVI plus linear lesions or PVI plus additional CFAE ablation-so called “spot ablation”-the overall recurrence rate was similar, but the mode of recurrence was different: regular AT was the prevailing type of arrhythmia in the spot ablation group (11 vs. 29%, p=0.03). Furthermore, Rostock et al, [12] published a stepwise approach of persistent atrial fibrillation, including PVI and extended lesions in left and right atrium. Following this strategy, 40% of patients developed ATs during the follow up.
Relatively few data is available in the literature regarding the incidence of LA ATs after antral PVI exclusively, in the absence of additional lines or ablation of CFAE. Our approach is the wide area circumferential antral PVI for treating patients suffering mainly from paroxysmal AF, using CT or MR image integration as well as intracardiac echocardiography (Fig. 1). The prevalence of left atrial AT is 11 % in our population which is more common than in the study by Wasmer et al. [13], who published a 4 % incidence in a very similar cohort recently.
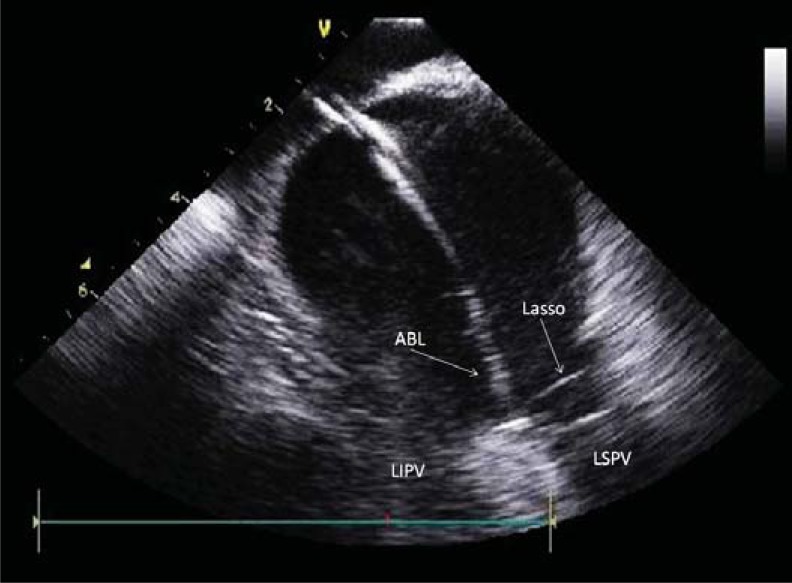
Fig. (1).
Intracardiac echocardiography image of left atrium. Ablation catheter (ABL) is sitting on the carina between left superior (LSPV) and left inferior (LIPV) pulmonary veins. Lasso: circular catheter in LSPV.
Organized ATs most commonly occurred several weeks to months after the AF ablation procedure, 8.8±7.7 months in our population, but sometimes during the initial attempt (either spontaneously or induced). Regarding the ATs induced immediately after the AF ablation the reported incidence was observed between 16%-36% [7, 14, 15]. One of these trials demonstrated that AT during the initial procedure was an independent predictor for an occurrence of late AT. Chang et al. showed [15] that elimination of all inducible ATs during the index procedure can decrease the incidence of late ATs (0.6%), suggesting that non-inducibility of AT can be an ablation endpoint. However, in contrast with this statement, other authors suggested that the mechanism of acute ATs is different from that of late ATs and may represent focal drivers that become manifest after elimination of higher frequencies and fibrillatory conduction in persistent AF population [16].
MECHANISM
Focal AT
The mechanism of atrial tachycardia varies with the ablation technique and to establish the exact mechanism of these tachycardias can be difficult and controversial which is especially true for the focal origin. A focal mechanism is usually suggested by the centrifugal activation pattern of the tachycardia on the electroanatomic maps. The mechanism most commonly is microreentry, non-reentrant types are usually caused by enhanced automaticity or triggered activity. The latter tachycardias are not so common; the prevalence probably does not exceed 10% of all ATs.
Following segmental pulmonary vein isolation and partially after wide area antral ablation with confirming electrical isolation these tachycardias are typically focal in nature and related to recovered PV conduction, [3, 17, 18]. In our series, it was 60 % of all ATs.
Centrifugal activation pattern does not exclude reentrant mechanism by itself. This pattern can also be obtained from the exit site of a slowly conducting isthmus of a reentrant circuit, especially if the resolution of mapping is not high enough. Gerstenfeld et al. performed a detailed pharmacologic and entrainment testing in a group of patients presenting focal ATs following PVI. These maneuvers demonstrated that most of these ATs were due to localized small reentry circuits anchored to the slow conduction areas caused by the previous ablation. Only one of six ATs showed typical focal characteristics suggesting non reentrant mechanism. [19]. Similarly, Deisenhofer reported a series of LA ATs, using PVI plus additional lines during the first procedure. More than one third of these tachycardias were due to small reentrant circuits related to reconnected PVs [6]. Shah et al. [20], demonstrated that 73% of ATs developing after AF ablation in their study were related to narrow, critical isthmuses at the vicinity of previously ablated PV ostial sites. Slow conduction in these areas was crucial for maintaining such a small reentrant circuit. These were 10-18 mm in size and produced rapid centrifugal activation in the rest of the LA. Interestingly, these critical isthmuses characterized by low amplitude-long duration intracardiac electrograms occupied almost half of the tachycardia cycle length and coincided with the isoelectric intervals in all 12 ECG leads, between flutter waves. (Fig. 2) These observations were highly consistent with the results of Yokokawa et al., [21] in terms of the observation that extremely slow conduction and adjacent anatomical barriers play a critical role in stabilizing these microreentrant circuits.
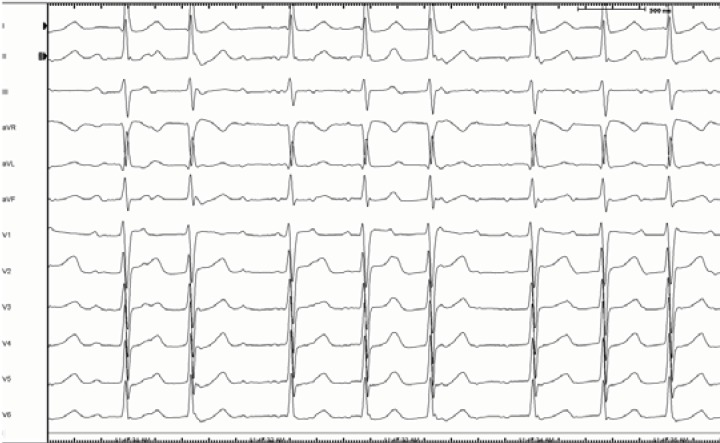
Fig. (2).
Twelve lead surface ECG of a narrow isthmus reentry, originating from the vicinity of right inferior pulmonary vein. Note the long isoelectric interval between discrete flutter waves.
In the case of pulmonary vein tachycardia the pathologic impulse originates from the PV myocardial cells, and activates the LA through even a single recovered gap within the original lesion set. In the vast majority of cases, the rhythm within the PV is faster than in the LA, but sometimes 1:1 conduction can occur. After closing the gap(s) between LA and PV, the tachycardia should continue as a dissociated rhythm within the PV (Fig. 3). The mechanism of these tachycardias is not clearly elucidated. Some data from the literature supported that the mechanism is non-reentrant due to enhanced automaticity and triggered activity but others demonstrated evidences suggestive of reentry inside the pulmonary veins [17, 19].
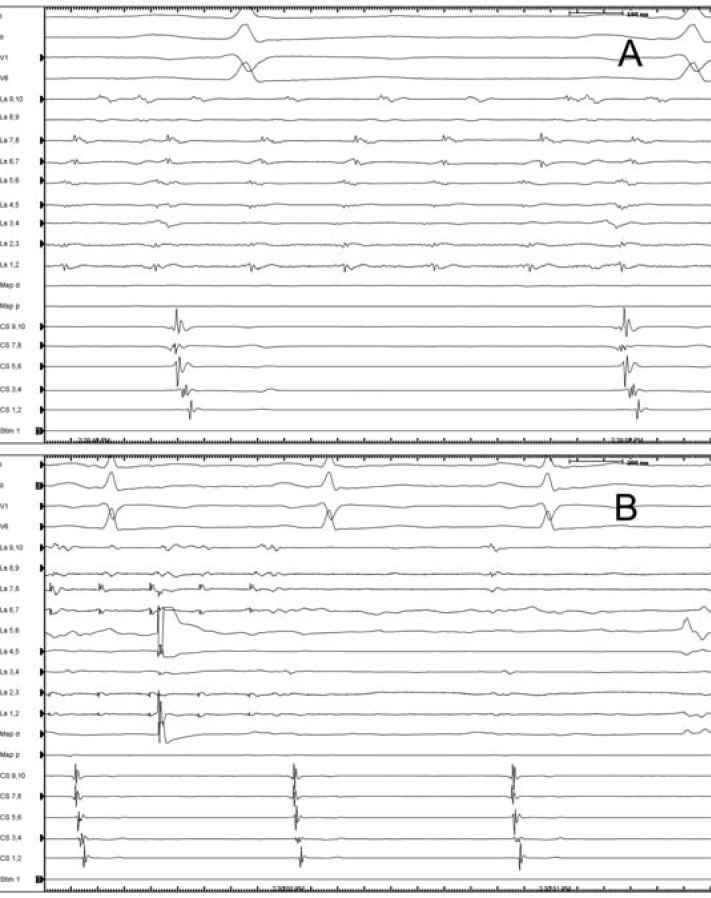
Fig. (3).
Rapid, regular pulmonary vein tachycardia persists within an isolated right superior pulmonary vein. The atria, outside the isolated vein are in sinus rhythm. (A). Radiofrequency ablation at the earliest spot within the vein, indicated by the ablation artifact on the 4-5 bipoles of Lasso catheter results in the termination of tachycardia. (B) Ls: lasso catheter, CS: coronary sinus catheter, Map d: mapping catheter.
Macroreentrant AT
Using anatomic ablation approaches with additional lines and/or CFAE ablation, the majority of ATs are macroreentrant, (73%-82%) which use regions of incomplete or recovered lesions and other anatomic obstacles. These tachycardias mostly revolve around the mitral valve (perimitral flutter, 28 % of cases in our experience) or less commonly around the isolated pulmonary venous ostia (roof dependent flutter, 12 % of cases), rarely around septal or posterior scars or LA appendage [18, 6]. The arrhythmia circuits of these ATs are usually independent from the PVs, but sometimes the myocardial sleeves within the veins can contribute to generating such a mechanism. Satomi et al. [22] as well as Robinson [23] presented clinical examples where the macroreentry circuits included the LA and PV myocardium as well, propagated via two conduction gaps located in the previous circular lesions which were relatively widely separated from each other (Fig. 4). It should be noted that most of these tachycardias presented with focal pattern on three dimensional electroanatomic maps (3D EAM), and could have been misdiagnosed as classical focal AT without detailed entrainment mapping guided by multipolar catheter within the PVs.
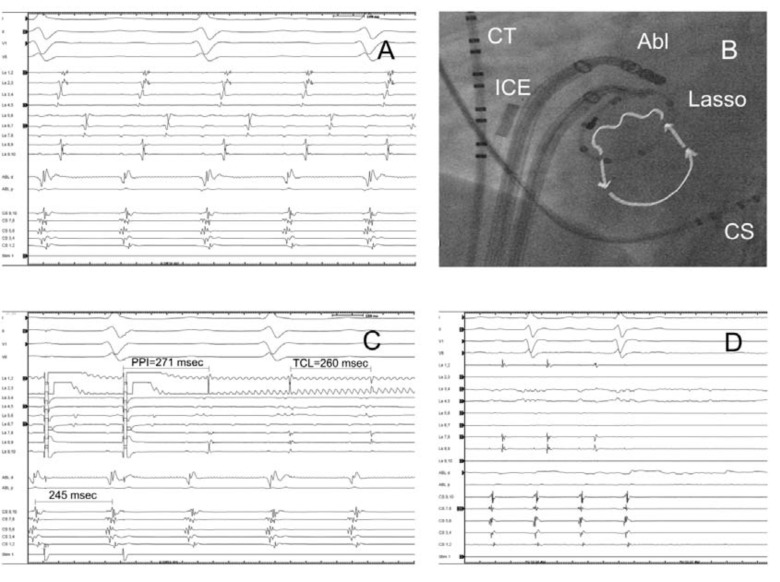
Fig. (4).
Gap related tachycardia originating from the circular lesion around the right inferior pulmonary vein. (A, 200mm/sec), and the corresponding catheter positions, arrows point out the direction of activation through two gaps (at lasso pole number 4-5 and 7-8) in the ablation line (B). Entrainment pacing with 245 msec from the earliest lasso bipole (4-5) showed concealed fusion and PPI-TCL was 11 msec. (C). Termination of tachycardia during ablation at the entrance (pole 4-5) (D,100 mm/sec). The vein was isolated with the second ablation at the pole 7-8 (not shown). RIPV: right inferior pulmonary vein, CT: crista terminalis catheter, ICE: intracardiac echocardiography probe, Abl: ablation catheter, Lasso: lasso catheter, CS: coronary sinus catheter, PPI: post pacing interval, TCL: tachycardia cycle length.
Patients may also experience typical, cavo-tricuspid isthmus dependent right atrial flutter after AF ablation, which is not unusual in this population. The published prevalence of typical flutter is between 15%-30% [24, 25].
CLINICAL MANAGEMENT
The time interval between the index procedure and the emergence of organized ATs differs in published series [3, 6, 7, 17] but most commonly occurs relatively early, several weeks to months after the AF ablation procedure, not rarely during the “blanking period”. Consequently, the initial treatment has to be conservative, considering that one third of ATs may resolve in time [7]. The mechanism of spontaneous restoration is not clearly elucidated, but probably related to healing and changing substrate following the ablation. The first task is to achieve an appropriate ventricular rate control, using beta blockers, digoxin or calcium channel blockers, but according to our observation, this strategy often fails. Electrical cardioversion can be more practical approach from the beginning, facilitated by using Class I or Class III antiarrhythmic agents, but-in agreement with Gerstenfeld et al.-if the tachycardia recurs beyond 2-3 months after first ablation, a repeated procedure is recommended [3].
Understanding the mechanism (see earlier) of the AT before the redo ablation procedure could facilitate the mapping and ablation strategy of the tachyarrhythmia. Twelve lead surface ECGs can be helpful to distinguish between macroreentrant and focal arrhythmias: continuous activation is typical for macroreentrant mechanism, whilst isoelectric baseline between P waves suggests focal arrhythmias [20]. As we already pointed out, in case of separated P waves with long isoelectric intervals in all 12 leads one can anticipate a narrow isthmus reentry, caused by little electrical activity of slowly conducting isthmuses, coinciding with isoelectric lines. Some authors demonstrated that focal AT tends to be faster, than macroreentrant tachycardias [26], but others reported the opposite [24] or found no difference between them [13]. P wave morphology can be useful to localize the source of the arrhythmia but this is very much dependent on preexisting scarring and the extent of previous ablation. This is why it might be more useful after AF ablation restricted to PVI, without substrate modification. Focal ATs originating from the proximity of PVs or inside the veins are typically positive in V1, and across the precordium. ATs from left PVs usually generate P waves that are flat in lead I, lead II<III, negative in aVL and showing M shape in V1 comparing to right venous origin which are mostly positive in lead I, lead II>III, and biphasic in aVL, with late positive peak in V1.
In the electrophysiology laboratory, during an ongoing AT, our approach is to use multipolar catheters in the right atrium and coronary sinus (CS) and try to entrain the tachycardia first from the cavo-tricuspid isthmus (CTI) to exclude the possibility of typical right atrial flutter. After LA ablation of AF, sometimes typical atrial flutter shows atypical ECG findings [27] as for instance upright flutter waves in the inferior leads, which incorrectly suggests left atrial origin. However, this pacing maneuver may prevent unnecessary instrumentation of left atrium.
After exclusion of CTI dependent flutter or other right atrial sources, entrainment pacing is suggested from the distal and proximal electrodes of coronary sinus catheter. If the post-pacing interval (PPI) minus tachycardia cycle length (TCL) is shorter than 30 msec. from those poles following termination of pacing which has resulted in atrial capture, the presence of perimitral flutter is very likely.
Pascale et al. [28] reported that the patterns and timing of CS activation provide a rapid stratification of most likely macroreentrant ATs. In patients with proximal to distal CS activation, the whole spectrum of possible AT mechanisms was observed and in distal to proximal CS activation the majority (61%) were clockwise peri-mitral AT. They described also other two CS activation patterns defined as “chevron” or “reverse chevron” when the activations recorded on both the proximal and distal CS dipoles were latest or earliest, respectively, and those patterns were associated mostly with roof-dependent macroreentry.
Once a left atrial access is obtained, atrial tachycardias can be mapped by endocardial activation mapping and/or entrainment mapping. To exclude focal tachycardias related to PV reconnection, mapping of the four pulmonary vein ostia using circular catheter is recommended. If the mechanism is small or narrow isthmus reentry - which confines usually to typical reconnection sites of PVs, (septal aspect of right, and anterior sites of left PVs) [29] - entrainment pacing will show intracardiac evidence of concealed fusion and PPI≈TCL from a limited area, demonstrating mid-diastolic or long fractionated electrograms. Using 3D EAM, small reentry is considered when the majority of the cycle length can be accounted for during mapping and the diameter of the circuit is<3 cm. Re-isolation of PV(s) usually leads to termination of tachycardias.
Focal non-reentrant atrial tachycardia shows radial spread of activation from a point source and the activation mapping fails to account for the majority of tachycardia cycle length [18]. Endocardial activation at the source is pre-systolic, generally limited to the second half of diastole. Clinically more often paroxysmal than persistent as opposed to reentrant rhythms typically show 20-40-msec variability in cycle length. If the PV origin can be excluded, activation mapping has to be extended to typical sites of focal AT in LA, like posterior wall, LA appendage, and mitral annulus.
The macroreentry is considered when the tachycardia is entrained with “in circuit” response from remote sites of LA, electrograms span all or most of the diastole, activation mapping accounts for at least 85% of the tachycardia cycle length, as well as the diameter of the reentry circuit is >3 cm, and continuous propagation sequence with earliest and latest activation adjacent to each other on the EAM [24, 30].
The most common form is the peri-mitral flutter traversing the mitral isthmus.
In case of single loop peri-mitral circuit, there are several options to produce linear ablation lesions to terminate the tachycardia. The most common choice is the classical mitral isthmus line between the left inferior PV and the posterior mitral annulus (MA). Usually, it is a fairly long and concave isthmus, where one can have difficulties getting good contact without using intracardiac echocardiography. Histological data confirmed that coronary sinus muscle sleeves in this location are present in up to 75% of patients, and extend onto LA isthmus musculature. Not surprisingly, coronary sinus ablation is required in at least 70% of patients but bidirectional block cannot always be achieved [31]. Another possibility is to create linear lesion from the right inferior PV to posterior MA, which is one the most difficult isthmuses because of the proximity of three different anatomical structures (right and left atrium and coronary sinus) and the slow pathway region. Next option is drawing lines from the right superior PV to anterior MA. This isthmus is usually longer than the posterior mitral isthmus, and this line passes the insertion of Bachmann bundle, which is relatively thick and may prevent the creation of a transmural lesion (Fig. 5). If we have multiloop circuits around the MA and right PVs, usually separate ablation lines are required to interrupt separate circuits, because of the aforementioned complexity of right posterior isthmus. In contrast, circuits around the MA and left PVs are much easier to ablate targeting the single left sided isthmus.
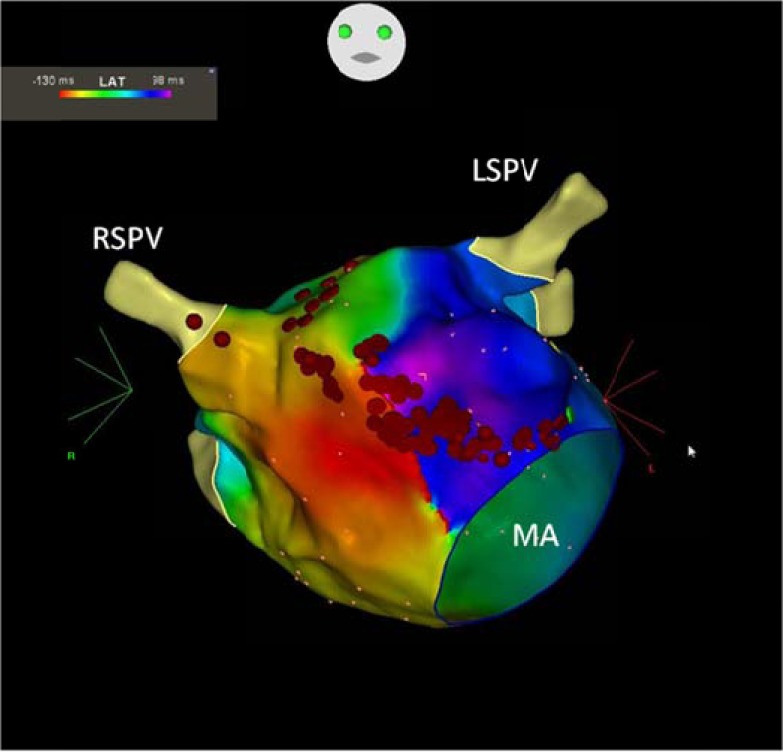
Fig. (5).
Perimitral reentry propagating around the mitral valve in counterclokwise direction on three dimensional CARTO activation map. Red dots represent the ablation line which was created between the anterior mitral annulus and the right superior pulmonary vein. MA: mitral annulus, RSPV: right superior pulmonary vein, LSPV: left superior pulmonary vein.
Completeness of mitral isthmus line is probably not always required for treating the tachycardia, but necessary to prevent them. Several studies pointed out that failure to achieve bidirectional mitral isthmus block increased the risk of developing left atrial flutter. Anouseh et al. demonstrated [32] that in patients in whom mitral isthmus block was not achieved, there was a fourfold risk of occurrence of subsequent ATs. Another question is whether these tachycardias are due to originally incomplete lines only or recovery of conduction. Sawney and co-authors [33] found that recovery of mitral isthmus conduction after initial achievement of block is common, 73 % and is associated with the emergence of peri-mitral flutter in 32%. They concluded that using electrophysiological endpoints alone might not be enough for maintaining long term results and at least in some patients it would be best to avoid mitral isthmus ablation if possible.
Macroreentry tachycardias around the isolated right of left side veins are less common, and in most patients completion of LA roof line leads to termination of tachycardias (Fig. 6).
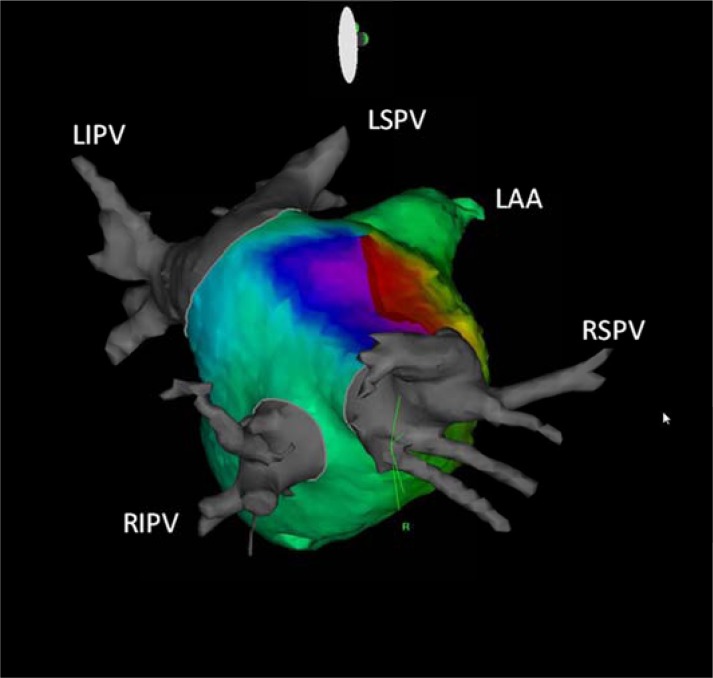
Fig. (6).
Roof dependent flutter propagating around the right sided pulmonary veins on three dimensional CARTO activation map with CT integration in right lateral view. RSPV: right superior pulmonary vein, RIPV: right inferior pulmonary vein, LSPV: left superior pulmonary vein, LIPV: left inferior pulmonary vein, LAA: left atrial appendage.
Regarding the practical aspects of ablation process, either during the mapping or pacing, the tachycardia can be terminated or transformed to another tachycardia(s), which complicates the procedure significantly. If such changes are predictable, isolation of reconnected PVs first is acceptable, since this is one of the major aims of procedure to prevent AF recurrence. Also, organized ATs related to gaps around PVs will be eliminated by this approach. Following the isolation of PVs if the tachycardia still persists or is inducible, entrainment and electroanatomic mapping can be carried out again, or otherwise empiric lesions can be applied.
ACKNOWLEDGEMENTS
Declared none.
SUMMARY
ATs occur relatively frequently after AF ablation procedures. The incidence of these tachycardias varies in the context of previous lesion set and probably of the extent of abnormal atrial electrophysiologic substrate. The mechanism in vast majority of cases is reentry related to gaps in prior ablation lines. Conservative therapy is usually not effective; radiofrequency ablation procedure is mostly successful but can be challenging and requires a complex approach.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Oral H, Knight BP, Tada H , et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–81. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Knight BP, Morady F. Left atrial flutter after segmental ostial radiofrequency catheter ablation for pulmonary vein isolation. Pacing Clin Electrophysiol. 2003;26:1417–9. doi: 10.1046/j.1460-9592.2003.t01-1-00202.x. [DOI] [PubMed] [Google Scholar]
- 3.Gerstenfeld EP, Callans DJ, Dixit S , et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation. 2004;110:1351–7. doi: 10.1161/01.CIR.0000141369.50476.D3. [DOI] [PubMed] [Google Scholar]
- 4.Oral H, Scharf C, Chugh A , et al. Catheter ablation for paroxysmal atrial fibrillation Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 5.Gaita F, Caponi D, Scaglione M , et al. Long-term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circ Arrhythmia Electrophysiol. 2008;1:269–75. doi: 10.1161/CIRCEP.108.774885. [DOI] [PubMed] [Google Scholar]
- 6.Deisenhofer I, Estner H, Zrenner B , et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation Incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace. 2006;8:573–82. doi: 10.1093/europace/eul077. [DOI] [PubMed] [Google Scholar]
- 7.Chugh A, Oral H, Lemola K , et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2:464–71. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Karch MR, Zrenner B, Deisenhofer I , et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation a randomized comparison between 2 current ablation strategies. Circulation. 2005;111:2875–80. doi: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney N, Anousheh R, Chen W , et al. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243–8. doi: 10.1161/CIRCEP.109.924878. [DOI] [PubMed] [Google Scholar]
- 10.Nademanee K, McKenzie J, Kosar E , et al. A new approach for catheter ablation of atrial fibrillation mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 11.Estner HL, Hessling G, Biegler R , et al. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation-a prospective randomized study. Pacing Clin Electrophysiol. 2011;34(8):939–48. doi: 10.1111/j.1540-8159.2011.03100.x. [DOI] [PubMed] [Google Scholar]
- 12.Rostock T, Drewitz I, Steven D , et al. Characterization, mapping, and catheter ablation of recurrent atrial tachycardias after stepwise ablation of long-lasting persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:160–9. doi: 10.1161/CIRCEP.109.899021. [DOI] [PubMed] [Google Scholar]
- 13.Wasmer K, Mönnig G, Bittner A , et al. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm. 2012;9(10):1660–6. doi: 10.1016/j.hrthm.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Daoud EG, Weiss R, Augostini R , et al. Proarrhythmia of circumferential left atrial lesions for management of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:157–65. doi: 10.1111/j.1540-8167.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang SL, Lin YJ, Tai CT , et al. Induced atrial tachycardia after circumferential pulmonary vein isolation of paroxysmal atrial fibrillation electrophysiological characteristics and impact of catheter ablation on the follow-up results. J Cardiovasc Electrophysiol. 2009;20(4):388–94. doi: 10.1111/j.1540-8167.2008.01358.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Chugh A, Ulfarsson M , et al. Relationship between the spectral characteristics of atrial fibrillation and atrial tachycardias that occur after catheter ablation of atrial fibrillation. Heart Rhythm. 2009;6(1):11–7. doi: 10.1016/j.hrthm.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F, Antz M, Ernst S , et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins lessons from double Lasso technique. Circulation. 2005;111:127–35. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 18.Mesas CE, Pappone C, Lang CC , et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation electroanatomic characterization and treatment. J Am Coll Cardiol. 2004;44:1071–9. doi: 10.1016/j.jacc.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 19.Gerstenfeld EP, Callans DJ, Sauer W , et al. Reentrant and nonreentrant focal left atrial tachycardias occur after pulmonary vein isolation. Heart Rhythm. 2005;2(11):1195–202. doi: 10.1016/j.hrthm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Shah D, Sunthorn H, Burri H , et al. Narrow, slow-conducting isthmus dependent left atrial reentry developing after ablation for atrial fibrillation ECG characterization and elimination by focal RF ablation. J Cardiovasc Electrophysiol. 2006;17(5):508–15. doi: 10.1111/j.1540-8167.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 21.Yokokawa M, Latchamsetty R, Ghanbari H , et al. Characteristics of atrial tachycardia due to small vs large reentrant circuits after ablation of persistent atrial fibrillation. Heart Rhythm. 2013;10(4):469–76. doi: 10.1016/j.hrthm.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Satomi K, Bänsch D, Tilz R , et al. Left atrial and pulmonary vein macroreentrant tachycardia associated with double conduction gaps a novel type of man-made tachycardia after circumferential pulmonary vein isolation. Heart Rhythm. 2008;5(1):43–51. doi: 10.1016/j.hrthm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Robinson T, Kalman JM. Proarrhythmia following prior pulmonary vein isolation what is the mechanismκ. J Cardiovasc Electrophysiol. 2012;23(8):884–6. doi: 10.1111/j.1540-8167.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- 24.Chae S, Oral H, Good E , et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50(18):1781–7. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Nam GB, Jin ES, Choi H , et al. Mechanism of regular atrial tachyarrhythmias during combined pulomonary vein isolation and complex fractionated electrogram ablation in patients with atrial fibrillation. Circ J. 2010;74(3):434–41. doi: 10.1253/circj.cj-09-0622. [DOI] [PubMed] [Google Scholar]
- 26.Gerstenfeld EP, Marchlinski FE. Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm. 2007;4(3) Suppl :S65–72. doi: 10.1016/j.hrthm.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Chugh A, Latchamsetty R, Oral H, Elmouchi D , et al. Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation. 2006;113:609–15. doi: 10.1161/CIRCULATIONAHA.105.580936. [DOI] [PubMed] [Google Scholar]
- 28.Pascale P, Shah AJ, Roten L , et al. Pattern and timing of the coronary sinus activation to guide rapid diagnosis of atrial tachycardia after atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2013;6(3):481–90. doi: 10.1161/CIRCEP.113.000182. [DOI] [PubMed] [Google Scholar]
- 29.Hall B, Jeevanantham V, Simon R , et al. Variation in left atrial transmural wall thickness at sites commonly targeted for ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;17:127–32. doi: 10.1007/s10840-006-9052-2. [DOI] [PubMed] [Google Scholar]
- 30.Kistler P, Sandler P, Fynn SP , et al. Electrophysiologic and electrocardiographic characteristics of focal atrial tachycardias originating from the pulmonary veins acute and long-term outcomes of radiofrequency catheter ablation. Circulation. 2003;108:1968,–75. doi: 10.1161/01.CIR.0000095269.36984.75. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo S, Wright M, Knecht SM, et al. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm. 2010;7(1):2–8. doi: 10.1016/j.hrthm.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Anousheh R, Sawhney NS, Panutich M , et al. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2010;33(4):460–8. doi: 10.1111/j.1540-8159.2009.02625.x. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney N, Anand K, Robertson CE , et al. Recovery of mitral isthmus conduction leads to the development of macro-reentrant tachycardia after left atrial linear ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4(6):832–7. doi: 10.1161/CIRCEP.111.964817. [DOI] [PubMed] [Google Scholar]