Figure 1.
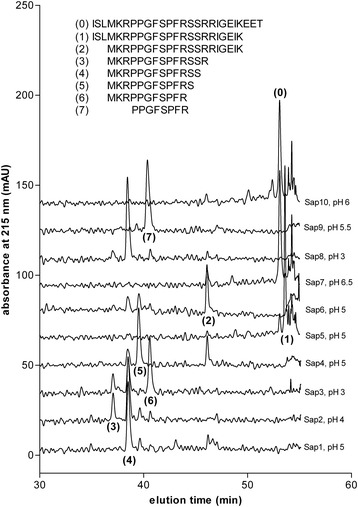
HPLC/MS characteristics of the Sap-catalyzed cleavage of the HK-D4 peptide. Ten μM of the synthetic peptide HK-D4 (which has the ISLMKRPPGFSPFRSSRIGEIKEET amino acid sequence) were treated with recombinant Sap1–10 in citrate (50 mM) or phosphate buffers (25 mM) at the optimal pH for the general proteolytic activity [26] of each individual Sap (specified in the figure) at an enzyme:substrate molar ratio of 1:50 for 24 hours at 37°C. The reaction was stopped using HCl, and the samples were analyzed using reversed-phase HPLC on an Eurosil Bioselect 300–5 C-18 column (Knauer) in a TFA-water-ACN binary gradient system. The fractions, which were collected at the major absorbance peaks (215 nm), were evaporated and analyzed using ESI-MS/MS in order to determine their amino acid sequence.
