The highly conserved abundant nuclear protein poly (ADP-ribose) polymerase-1 (PARP-1) is activated by DNA damage. PARP-1 activation is associated in DNA repair, cell death and inflammation. Since oxidative stress induced robust DNA damage and wide spread inflammatory responses are common pathologies of various CNS diseases, the attention towards PARP-1 as a therapeutic target has been amplifying. This review highlights the multiple roles of PARP-1 in neurological diseases and potential of PARP-1 inhibitors to enter clinical translation.
Activity of PARP-1 in physiology and patho-physiology: PARP-1 is an abundant nuclear enzyme consisting of three domains: N-terminal DNA binding domain containing two zinc fingers, auto-modification domain and catalytical domain. PARP-1 functions as a sensor of DNA damage and to bind to DNA breaks/nicks through Zn finger domains. Upon activation PARP-1 synthesizes poly ADP-ribose polymers by catalyzing nicotinamide adenine dinucleo tide (NAD+) into nicotinamide and ADP-ribose, which are then used as substrates to form linear or branched polymers (poly(ADP-riboses); PARs). These polymers of ADP-ribose units then covalently attached to Glu, Lys or Asp residues of acceptor proteins (heteromodification) or onto PARP1 itself (automodification). The high negative charge of PAR dramatically affects the function of target proteins, leading to electrostatic repulsion among histone proteins and DNA, a process implicated in chromatin remodeling, DNA repair and transcriptional regulation. However, the covalent modification of proteins by the transfer of ADP-ribose residues is only momentary due to the rapid action of a group of enzymes including, poly(ADP-ribose) glycohydrolase (PARG), ADP-ribosyl hydrolase3 (ARH3), nucleoside diphosphate linked to another moiety X (NUDIX) and macrodomain containing proteins (MDCPs) which catalyze the hydrolysis of these polymers into free ADP-ribose (ADPR) units (Sriram et al., 2014). Besides to the inception DNA repair mechanism, PARP-1 activity is found to be vital for epigenetic regulation of mitochondrial DNA repair and transcription as well (Lapucci et al., 2011). Furthermore, the activity of PARP-1 is also essential for a vital epigenetic mechanism, DNA methylation (Lodhi et al., 2014).
PARP-1 acts at the center of cellular stress. Oxidative stress causes DNA damage and consequently activates PARP-1 to repair the damaged DNA (Krietsch et al., 2012). PARP-1 has been implicated in neuronal pathology, as the brain is highly susceptible to oxidative stress. The degree of the PARylation in response to DNA damage largely depends on the nature and amount of DNA breaks produced. For low levels of DNA damage, PARP1 activity favors repair and survival by interacting with DNA repair enzyme cascade, such as such as XRCC1 and DNA-dependent protein. Moderate DNA damage leads to apoptotic cell death, during which PARP1 will be cleaved into two fragments by caspases. Cleavage of PARP1 is assumed to foil the activation of PARP1 by DNA damage and thereby it prevents cells from pathological consequences such as necrosis of cells. For extensive DNA injury as observed during ischemia/reperfusion and inflammatory conditions, the massive production of PAR ultimately causes cell-death via at least two distinct mechanisms: energy-failure induced necrosis or apoptosis-inducing factor (AIF) dependent apoptosis (Sriram et al., 2014).
In the cells with extensive DNA damage or damage that is not repaired, PARP1 remains activated, leading to continued NAD+ depletion and further ATP consumption in order to resynthe-size NAD+ (Berger et al., 1983). Continued NAD+ depletion has also been shown to induce a rapid mitochondrial dysfunction, which was followed by a collapse in mitochondrial potential, and the release of AIF and cytochrome c (Alano et al., 2010). The released AIF thus mediates the caspase-independent cell death termed parthanatos. In contrast to its name, AIF is now further acknowledged as a necrotic rather than an apoptotic mediator, providing further support for the necrotic role of PARylation. Thus it is can be inferred that inhibition of elevated PARylation could be beneficial (Figure 1) (Sriram et al., 2014).
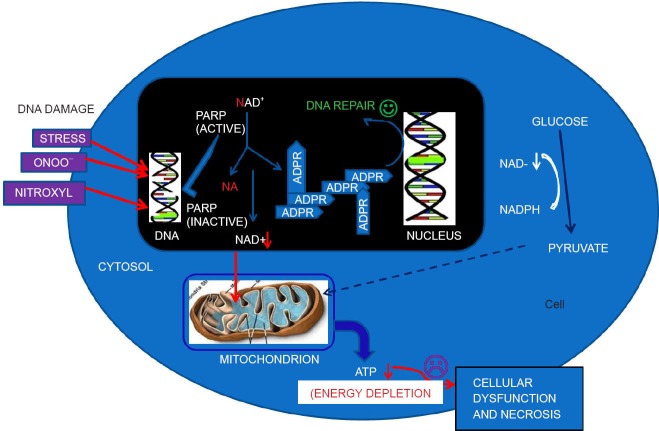
Figure 1.
Diagram representing the activity of ADP-ribosylation and consequences.
DNA-damaging stimuli (oxido-nitrosative stress) cause poly(ADP-ribose) polymerase (PARP) activation. Activated PARP cleaves nicotinamide adenine dinucleo tide (NAD) into nicotinamide and ADP-ribose and polymerizes the latter on nuclear-acceptor proteins. Poly(ADP-ribosylation) facilitates DNA repair and thus permits cell survival. Severe DNA damage, however, leads to overactivation of PARP, resulting in NAD and ATP depletion and necrotic cell death.
Impact of PARP-1 in neurological diseases: As aforesaid, PARP-1 has been implicated in neuronal pathology, as the brain is highly susceptible to oxidative stress. We can go for the inhibition of PARylation for minimizing or nullifying the harmful effects of elevated oxidative-nitrosative stress. PARP-1 is the main enzyme responsible for PARylation, so inhibiting this enzyme could be useful in this regard. In the laboratory there are different types of models (in vitro and in vivo) for studying the possible protective role of PARP-1 inhibition. In the in vivo models PARP-1 inhibition can be achieved in two ways either by using knockout animal models or by using chemical inhibition, while in vitro models equip the cell lines which are devoid of PARP-1 gene. As inferred theoretically, even the results from many studies (in vitro and in vivo) have revealed the promising neuro-protective role of PARP-1 inhibition (Sriram et al., 2014).
There are many neurological indications, in which PARP-1 has been studied extensively such as stroke, traumatic brain injury, neurodegenerative diseases (Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis) and neuro-inflmmatory diseases such as multiple sclerosis. Almost all the studies revealed a unique conclusion, that is targeting PARP-1 in neurological is a promising approach for minimizing the harmful effects of oxidative-nitrosative stress (Jangra et al., 2013; Martire et al., 2013; Rulten et al., 2014; Sriram et al., 2014).
Among the neurological diseases stroke is extensively studied indication for the impact of PARP-1. The results from many studies have proved the protective impact of PARP-1 inhibition in stroke. A recent break through study by Matsuura and group has further enriched the concept of protective role PARP-1 inhibition in stroke. They have reported the outstanding findings from their study of PARP-1 inhibitor, MP-124 in cynomolgus and rhesus monkeys. Even the results from that particular study are in compliant with recommendations laid by Stroke Therapy Academic Industry Roundtable (STAIR). The magnitude of the therapeutic effect, as well as the therapeutic window of intervention is important while dealing with cerebral stroke and ischemia. The therapeutic window of PARP-1 inhibitor is making them an attractive class of drugs for these indications, as a very few number of drugs show such a good therapeutic window. However, presently a phase-1 clinical study of PARP1 inhibitor, JPI-289 is underway for stroke (Matsuura et al., 2011; Moroni et al., 2012; Sriram et al., 2014). In addition, several PARP-1 inhibitors are in different clinical phases of development as a single and combined therapy for the cancer indications ranging from solid tumors to breast cancers (Sriram et al., 2014).
To be honest the exploration of impact PARP1 in the other central nervous system diseases is a bit late as compared to stroke. It is still better in the case of traumatic brain injury as compared to neurodegenerative diseases (Alzheimer's and Parkinson's) and neuroinflammatory diseases such as multiple sclerosis. Apart from stroke, the results from several studies of PARP-1 in different neurological models are also providing potential evidence of the approach (Sriram et al., 2014; Stoica et al., 2014)
Conclusion and future perspectives: Extreme severity and restricted clinical options are making neurological indications more difficult to handle. However, the treatment with PARP-1 inhibitors showing promising results for neurological indications in multiple preclinical studies. Now PARP-1 could be reliable target for newer drug developments in the field of neurological diseases. In spite of this much propitious situation, PARP-1 inhibition therapy has its own set of limitations. The primary function of PARP1 is in DNA-damage repair, widespread PARP-1 inhibition may leave cells with larger number of DNA anomalies which may amplify the risk of genomic instability. Surviving neurons with DNA damage might be dysfunctional and thus later on undergo apoptosis. Additionally, the prolonged PARP-1 inhibition might have negative effects beyond the genetic stability. In addition, at present the available drugs are not exceptionally specific for PARP-1. To combat these issues some more studies are still required to corroborate the safety of the therapeutic approach.
References
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N, Sims J, Catino D, Berger S. Poly (ADP-ribose) polymerase mediates the suicide response to massive DNA damage: studies in normal and DNA-repair defective cells. Princess Takamatsu Symp. 1983;13:219–226. [PubMed] [Google Scholar]
- Jangra A, Datusalia AK, Khandwe S, Sharma SS. Amelioration of diabetes-induced neurobehavioral and neurochemical changes by melatonin and nicotinamide: implication of oxidative stress–PARP pathway. Pharmacol Biochem Behav. 2013;114:43–51. doi: 10.1016/j.pbb.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Krietsch J, Caron MC, Gagné JP, Ethier C, Vignard J, Vincent M, Rouleau M, Hendzel MJ, Poirier GG, Masson JY. PARP activation regulates the RNA-binding protein NONO in the DNA damage response to DNA double-strand breaks. Nucleic Acids Res. 2012;40:10287–10301. doi: 10.1093/nar/gks798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapucci A, Pittelli M, Rapizzi E, Felici R, Moroni F, Chiarugi A. Poly(ADP-ribose) polymerase-1 is a nuclear epigenetic regulator of mitochondrial DNA repair and transcription. Mol Pharmacol. 2011;79:932–940. doi: 10.1124/mol.110.070110. [DOI] [PubMed] [Google Scholar]
- Lodhi N, Kossenkov AV, Tulin AV. Bookmarking promoters in mitotic chromatin: poly (ADP-ribose) polymerase-1 as an epigenetic mark. Nucleic Acids Res. 2014;42:7028–7038. doi: 10.1093/nar/gku415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire S, Fuso A, Rotili D, Tempera I, Giordano C, De Zottis I, Muzi A, Vernole P, Graziani G, Lococo E. PARP-1 modulates amyloid beta peptide-induced neuronal damage. PLoS One. 2013;8:e72169. doi: 10.1371/journal.pone.0072169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S, Egi Y, Yuki S, Horikawa T, Satoh H, Akira T. MP-124, a novel poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor, ameliorates ischemic brain damage in a non-human primate model. Brain Res. 2011;1410:122–131. doi: 10.1016/j.brainres.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Chiarugi A, Formentini L, Camaioni E, Pellegrini-Giampietro D, Chen Y, Liang S, Zaleska M, Gonzales C. Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly (ADP-ribose) polymerase. Br J Pharmacol. 2012;165:1487–1500. doi: 10.1111/j.1476-5381.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulten SL, Rotheray A, Green RL, Grundy GJ, Moore DA, Gómez-Herreros F, Hafezparast M, Caldecott KW. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res. 2014;42:307–314. doi: 10.1093/nar/gkt835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram CS, Jangra A, Kasalaa ER, Bodduluru LN, Bezbaruah BK. Targeting poly (ADP-ribose) polymerase1 in neurological diseases: a promising trove for new pharmacological interventions to enter clinical translation. Neurochem Int. 2014;76:70–81. doi: 10.1016/j.neuint.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Loane DJ, Zhao Z, Kabadi SV, Hanscom M, Byrnes K, Faden AI. PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J Neurotrauma. 2014;8:758–772. doi: 10.1089/neu.2013.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]