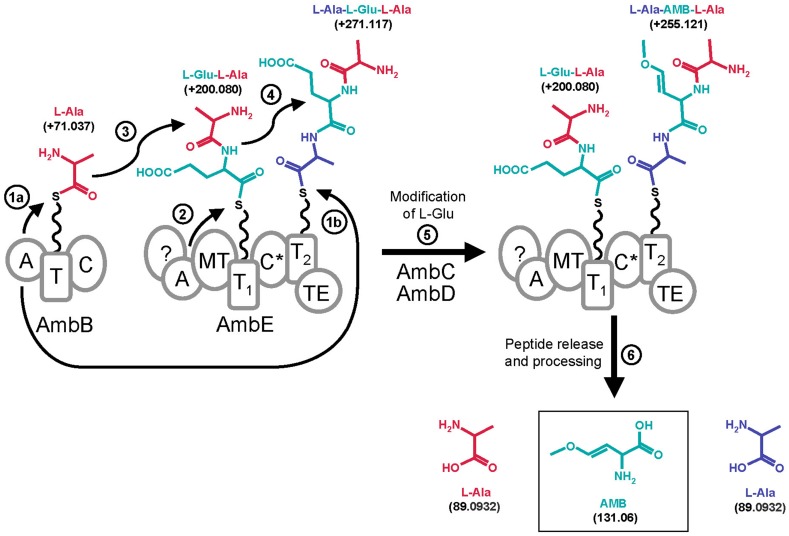
FIGURE 7.
Proposed model for AMB biosynthesis with indication of mass shifts (in Da) of tethered molecules. The A domain of AmbB activates L-Ala (residue shown in red) and loads it onto its own T domain (step 1a) and onto the T2 domain of AmbE (step 1b). The A domain of AmbE activates L-Glu (residue shown in blue) and loads it onto its T1 domain (step 2). Then, the C domain of AmbB or the C* domain of AmbE would condense L-Ala with L-Glu onto the T1 domain of AmbE forming the L-Glu-L-Ala dipeptide (step 3). This would subsequently be condensed with L-Ala on the T2 domain of AmbE to give the tripeptide L-Ala-L-Glu-L-Ala (step 4). Modification of the central L-Glu by action of AmbC, AmbD, the MT domain of AmbE, and eventually by the C* domain and/or by the N-term of AmbE, would give rise to the tripeptide L-Ala-AMB-L-Ala (step 5). This is believed to be the final peptide released from AmbE by action of the TE domain (step 6). Finally, the flanking L-Ala residues are removed by a processing step to yield AMB (step 6).