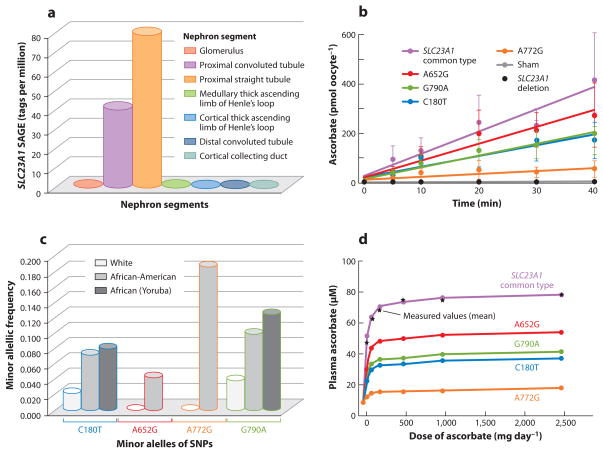
Figure 3.
SLC23A1 activity and human genetic variation. (a) Distribution of SLC23A1 in kidney nephron segments by Serial Analysis of Gene Expression (SAGE) libraries containing expression data from microdissected glomeruli and six different nephron segments; values are expressed as tags per million. (b) Effect of SLC23A1 SNPs on ascorbate transport. Xenopus laevis oocytes were microinjected with the following SLC23A1 cRNAs: common type; sham injected; human deletion construct; and SNPs A652G (rs34521685), G790A (rs33972313), A772G (rs35817838), and C180T (rs6886922). (c) Population prevalences of SLC23A1 polymorphisms. Shown are averaged minor allelic frequencies of SLC23A1 genotypes in African (n = 48), American African (n = 438), and white (n = 1,874) individuals, using pooled genotype data. (d) Modeled effects of SLC23A1 polymorphisms on plasma ascorbate concentrations in humans. Values in healthy young women for common type SLC23A1 are measured and calculated fasting steady-state plasma ascorbate concentrations. For women with SNPs, values are calculated from wild-type data comparisons to transport data in panel b. Reprinted with permission of the American Society for Clinical Investigations, from Corpe C.P., Tu H., Eck P., Wang J., Faulhaber-Walter R., Schnermann J., Margolis S., Padayatty S., Sun H., Wang Y., Nussbaum R.L., Espey, M.G., and Levine, M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. April 1:120(4). Copyright 2010 (17).