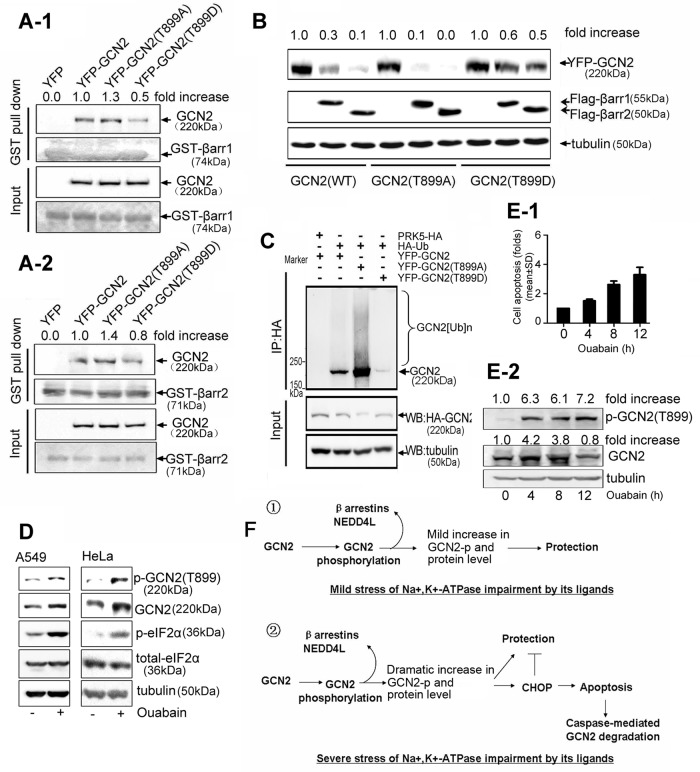
FIGURE 8:
Effect of GCN2 phosphorylation on ubiquitin-mediated GCN2 degradation. (A) HEK293 cells were transfected with YFP-tagged GCN2 (WT), GCN2 (T899A), GCN2 (T899D), or empty vector. Cell lysates were precipitated with glutathione beads incubated with purified GST–-β-arrestin1 (A-1) or GST–β-arrestin2 (A-2). Precipitates were analyzed by immunoblot analysis with the indicated antibodies. (B) A549 cells were cotransfected with YFP-tagged GCN2 (WT), GCN2 (T899A), or GCN2 (T899D) plus FLAG-β-arrestin1/2. After 36 h, lysates were examined by immunoblot analysis with the indicated IgGs. (C) A549 cells were cotransfected with HA-Ub and YFP-tagged GCN2 (WT), GCN2 (T899A), or GCN2 (T899D) and further treated with MG132 (50 μM) for 5 h before being harvested. Cells lysates were immunoprecipitated with anti-HA IgG, and the immune pellets were detected by immunoblot analysis with the indicated antibodies. (D) A549 and HeLa cells were treated in the presence or absence of ouabain at 100 nM for 12 h. Phosphorylation of GCN2 T899, phosphorylation of eIF2α, and the total eIF2α were assessed by immunoblot analysis. (E) A549 cells were treated with 400 nM ouabain for 0, 4, 8, and 12 h, respectively. Cell apoptosis was detected by flow cytometry. Data are expressed as the fold of cell apoptosis in ouabain-treated cells relative to control cells (E-1).The lysates were detected by immunoblot analysis with the indicated antibodies. For the quantitation of the band densities of immunoblot, values were calculated as the ratio of p-GCN2 (T899) or GCN2 to tubulin and normalized to the values obtained in control cells (1.0; E-2). A representative immunoblot analysis for each treatment from three independent experiments is shown. (F) Regulatory roles of GCN2 in cells sensing the mild or severe stress of Na+,K+-ATPase impairment by its ligands.