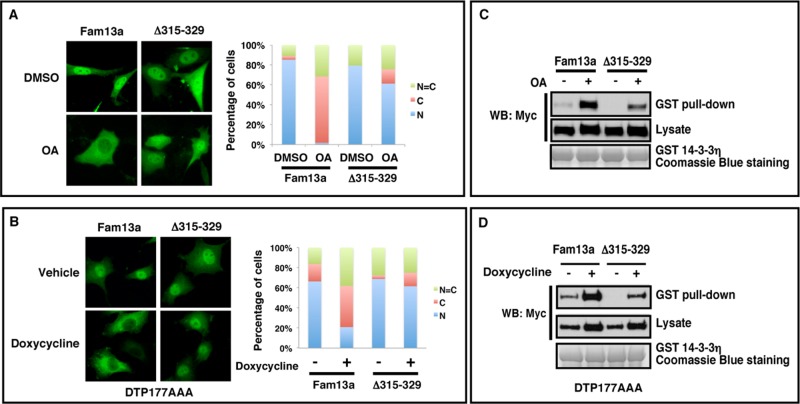
FIGURE 4:
The 14-3-3 binding domain is important for cytoplasmic sequestration of Fam13a in response to PP2A inhibition. (A) Representative immunofluorescence images, showing the subcellular distribution of Fam13a and Δ315–329 in control (DMSO) and OA-treated NIH3T3 cells. Fam13a and Δ315–329 were primarily nuclear in control (DMSO) NIH3T3 cells. OA treatment induced cytoplasmic localization of Fam13a. Δ315–329 remained nuclear in the majority of OA-treated cells. (B) Representative immunofluorescence images, showing subcellular distribution of Fam13a and Δ315–329 in control (vehicle-treated) and doxycycline-treated Aα exchange (DTP177AAA) cells. Fam13a and Δ315–329 were nuclear in vehicle-treated cells. After doxycycline treatment, Fam13a was mislocalized. Δ315–329 remained nuclear in the majority of treated cells. Bar graphs in A and B indicate the percentage of cells that exhibit nuclear, cytoplasmic, or homogeneous subcellular distribution. (C) The 14-3-3 binding motif (residues 315–329) is required for the effect of PP2A inhibition on binding between Fam13a and bacterially expressed GST–14-3-3η in a GST pull-down assay. Fam13a- and Δ315–329–expressing cells were treated with DMSO or OA. Cell lysates were used for the GST pull-down assay. Note that OA treatment significantly enhanced interaction between Fam13a and GST–14-3-3. Compared to wild-type Fam13a, Δ315-329 showed much weaker interaction with 14-3-3 after OA treatment. (D) The 14-3-3 binding motif (residues 315–329) is required for the effect of B56s knockdown on binding between Fam13a and GST–14-3-3 in a GST pull-down assay. Fam13a- and Δ315–329–expressing Aα exchange cells were treated with vehicle or doxycycline. Cell lysates were used in a GST pull-down assay. Doxycycline treatment significantly enhanced the interaction between Fam13a and 14-3-3. However, Δ315–329 showed weaker interaction with 14-3-3 compared with wild-type Fam13a after doxycycline treatment.