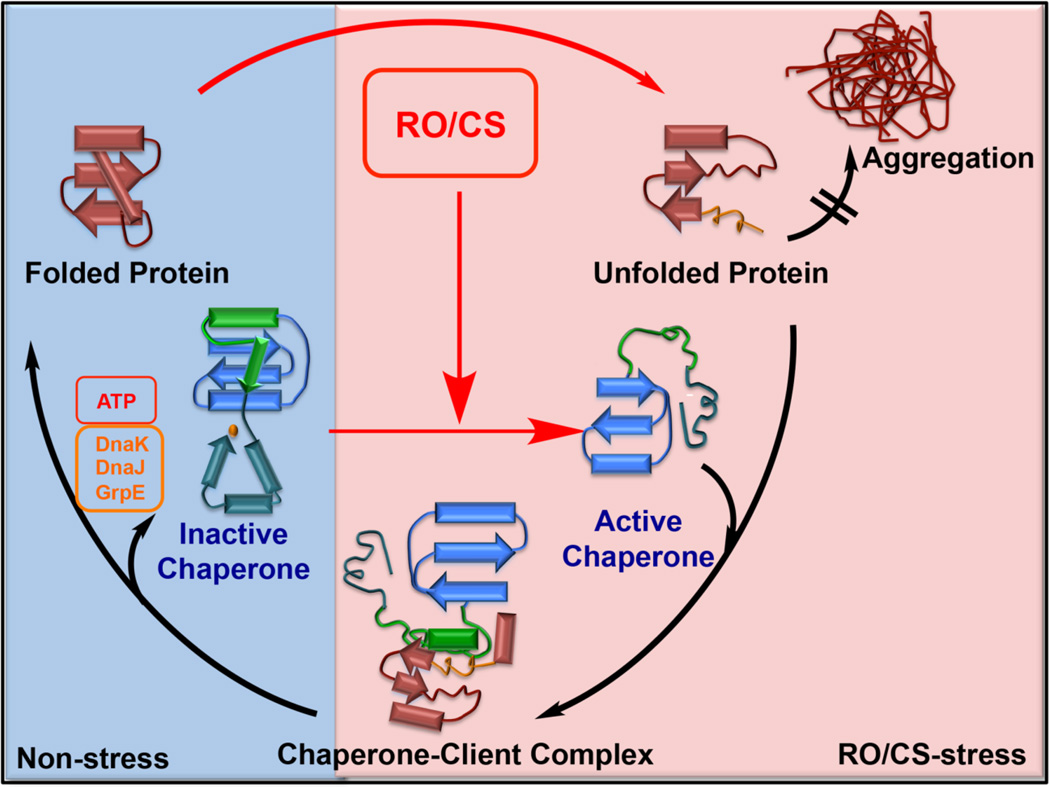
Figure 3. Redox-mediated activation of conditionally disordered chaperones.
RO/CS can cause substantial structural changes in proteins, leading to protein unfolding and aggregation. To sequester unfolding intermediates and prevent accumulation of toxic protein aggregates, bacteria employ the stress-specific molecular chaperone Hsp33. Under non-stress conditions, Hsp33 is well-folded and chaperone-inactive. Its four conserved cysteine residues are reduced and involved in high affinity binding of zinc. Upon exposure to oxidative protein unfolding conditions, Hsp33 undergoes oxidative disulfide bond formation, zinc release and massive structural rearrangements, including significant protein unfolding. In this partially intrinsically disordered conformation, Hsp33 is chaperone-active and able to interact with partially unfolded protein intermediates. Upon return to non-stress conditions, the disulfide bonds are reduced and the client proteins are transferred to the DnaK/DnaJ/GrpE system, which uses ATP to refold the client proteins. Hsp33 is specific for prokaryotes and unicellular eukaryotes. Very recently, Get3 has been identified to serve as the likely functional analogue of Hsp33 in yeast.