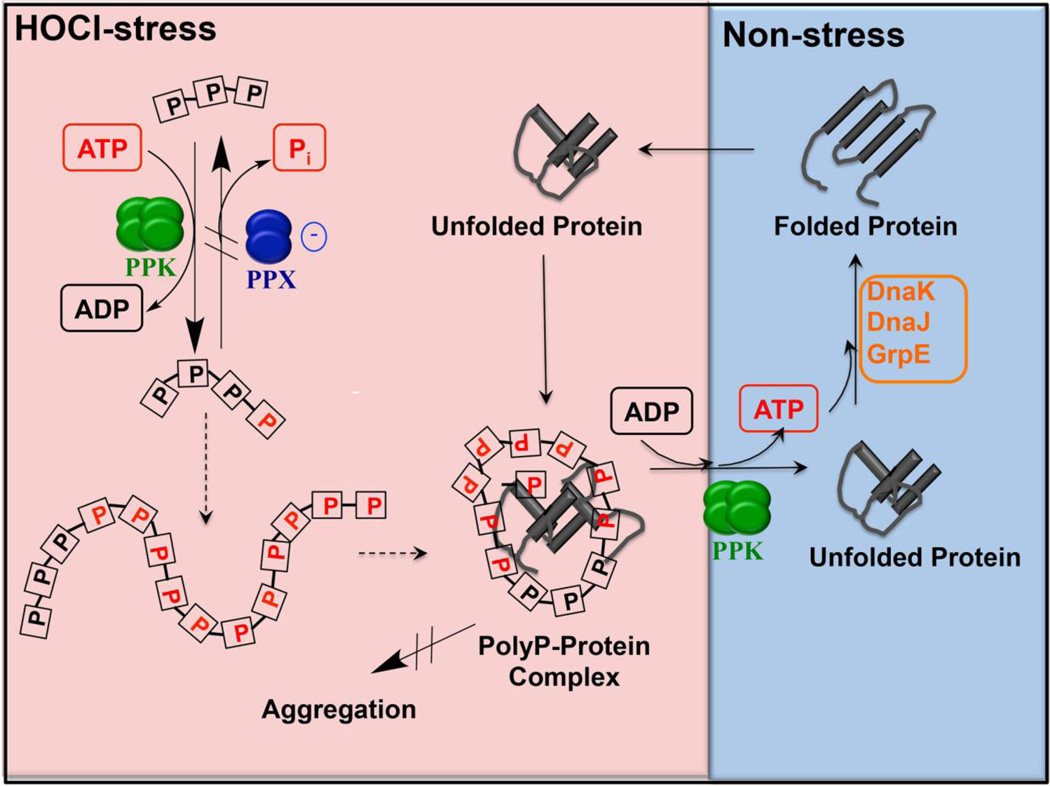
Figure 4. Model of polyP’s chaperone function.
Treatment of bacteria with HOCl leads to the conversion of cellular ATP into long chains of polyphosphate (polyP) catalyzed by the conserved enzyme polyphosphate kinase (PPK). PolyP accumulation in the cell is a consequence of the reversible oxidative inactivation of the polyP-degrading enzyme polyP phosphatase (PPX), which contains an oxidation-sensitive cysteine in its polyP-binding site. PolyP accumulation results in a significant depletion of the cellular ATP level, affecting most ATP-dependent cellular processes, including ATP-depending chaperones, such as the DnaK/DnaJ/GrpE system. PolyP is able to compensate for the lack of ATP-dependent chaperones by serving as a scaffold that binds to unfolding proteins, preventing them from aggregation, and keeping them soluble and refolding competent. Once reducing conditions are restored, polyP is reconverted to ATP and client proteins are released. Reactivated ATP-dependent foldases then support the refolding of these client proteins.