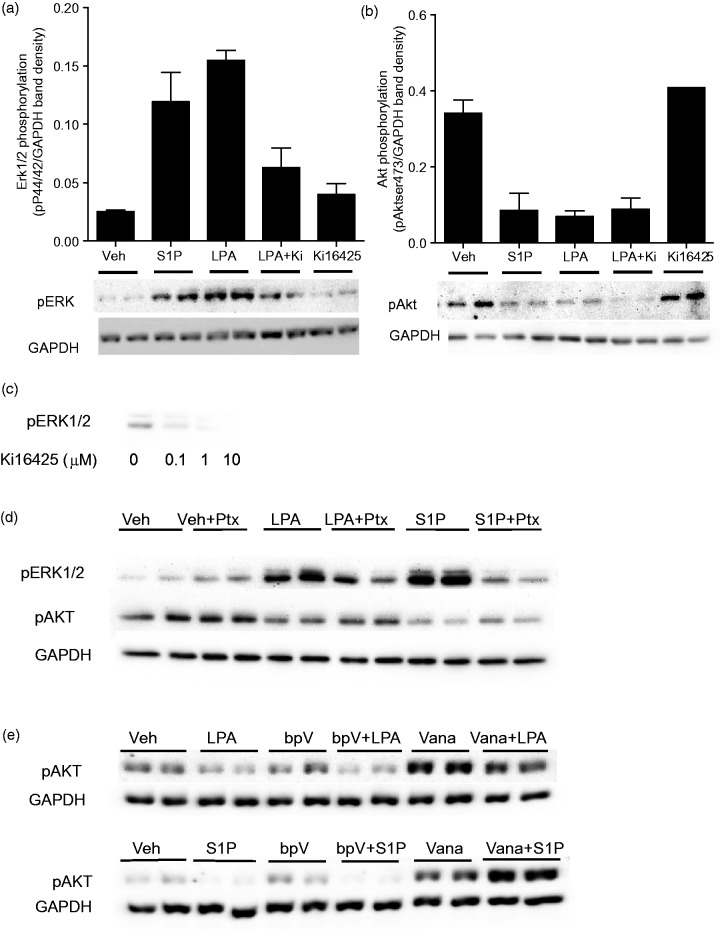
Figure 6.
LPA and S1P decrease Akt phosphorylation and increase Erk phosphorylation in hNP cells.
hNP cells were grown in the absence of bFGF for 24 hr and treated with the indicated compounds in fresh media. (a) Cells were treated with 0.1 µM S1P, 1 µM LPA, or 10 µM Ki16425 for 10 min and then harvested for Western blotting analysis with anti-phospho p42/44 Erk Map kinase antibodies. Expression of GAPDH protein was determined as a housekeeping standard. Band intensities were quantified and Erk phosphorylation levels were normalized to GAPDH levels. (b) Cells were treated with 0.1 µM S1P, 1 µM LPA, or 10 µM Ki16425 for 30 minutes, and then harvested for Western blotting analysis with anti-phospho serine 473 Akt kinase antibodies. Expression of GAPDH protein was determined as a housekeeping standard. Band intensities were quantified and Erk phosphorylation levels were normalized to GAPDH levels. (c) hNP cells were grown in the absence of bFGF for 24 hr and treated with the indicated concentrations of Ki16425 in the absence of exogenous LPA for 10 minutes. Cells were harvested and analyzed by Western blotting analysis with anti-phospho p42/44 Erk Map kinase antibodies. (d) hNP cells were treated in duplicate with 1 µM LPA or 0.1 µM S1P with or without pretreatment with 100 ng/mL pertussis toxin (Ptx) for 12 hr. ERK1/2 phosphorylation was assessed after 10-min LPA/S1P treatment, and Akt phosphorylation was assessed after 30-min LPA/S1P treatment. (e) hNP cells were treated in duplicate with 1 µM LPA or 0.1 µM S1P with or without 30-min pretreatment with 2.5 µM bpV(OHpic) or 100 µM sodium vanadate. ERK1/2 phosphorylation was assessed after 10-min LPA/S1P treatment, and Akt phosphorylation was assessed after 30-min LPA/S1P treatment.