Figure 3.
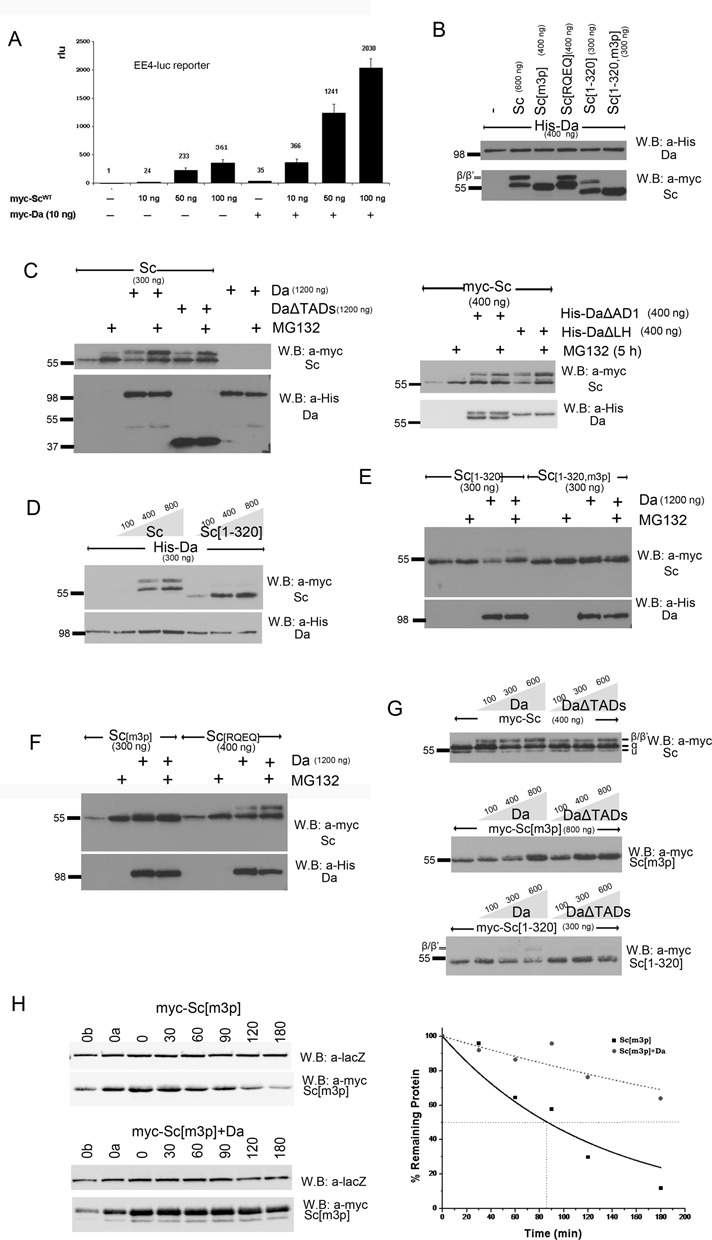
Proteasomal degradation of Sc in the presence of Da depends on phosphorylation of Sc, its DNA binding ability and on the AD1 domain of Da. (A) Luciferase assays on transiently transfected S2 cells with an EE4-luc reporter. Activation levels by increasing amounts of myc-Sc in the presence or absence of co-transfected Da. Relative luciferase units (rlu) are shown and are normalized on the activity of the reporter gene alone, set to 1. Averages/standard deviations of triplicates are shown. In all subsequent panels N-terminally 3xmyc-tagged Sc proteins or His-tagged Da were detected. The amount loaded was adjusted according to the activity of luciferase expressed from control plasmids (Ract-luc) transfected at the same time as the indicated plasmids (to measure transfection efficiency). (B) Whole cell extracts from S2 cells co-transfected with expression constructs as indicated. Note that Da co-expression causes the appearance of new Sc bands that run about 5 kd above the major band. The triple phosphomutant (m3p) abolishes this modification. (C) S2 cells transfected with the indicated constructs were treated (+) or not (-) with MG132 (for ∼5 h) before lysis. Proteasome inhibition increased the levels of Sc when it was expressed alone or together with Da. However, upon co-expression with DaΔTADs, only the β/β’ forms showed significant stabilization. Proteasome inhibition did not seem to increase the levels of Da (lower panel). The same experiment was done using deletion variants DaΔAD1 and DaΔLH (right panel). Proteasome inhibition increased the levels of Sc when it was expressed alone or together with DaΔLH. However, upon co-expression with DaΔAD1, only the β/β’ forms showed significant stabilization. Proteasome inhibition did not seem to increase the levels of Da variants (lower panel). (D) Blots from S2 cell extracts transfected with Da and increasing amounts of Sc or Sc[1–320]. Note that increasing Sc or Sc[1–320] did not diminish the levels of Da; if anything, it caused a small upregulation. (E,F) Cells transfected with Sc[1–320], Sc[1–320, m3p], Sc[m3p] and Sc[RQEQ] in the absence or presence of His-Da were treated or not with MG132. Note that proteasome inhibition stabilized solo Sc[m3p] and Sc[RQEQ], but had no effect when Da is co-expressed (D). Sc[1–320] solo was barely stabilized by MG132 treatment, but when Da was co-expressed MG132 stabilized Sc[1–320]. Finally, MG132 did not seem to increase the levels of Sc[1–320, m3p], whether expressed alone or with Da. (G) Steady-state levels of Sc, Sc[m3p] or Sc[1–320] with increasing amounts of Da variants. Sc[m3p] levels increased with increasing Da or DaΔTADs. Sc[1–320] levels dropped with increasing Da, but were not affected by DaΔTADs. For comparison, Sc levels were not significantly affected by either Da or DaΔTADs; if anything, a weak stabilization at low Da levels was observed. Note that the β form is much more prominent for Sc than for Sc[1–320] and is absent in Sc[m3p]. (H) Degradation kinetics of myc-Sc[m3p] transfected alone or co-transfected with Da after protein synthesis inhibition by cycloheximide. Co-transfected β-galactosidase (lacZ, a stable protein) was used as a loading control. 0, 30,…, 180 refer to minutes after cycloheximide addition. 0a, 0b: 1/2 or 1/4, respectively, of input (0 min) for densitometry calibration. One indicative blot and densitometry plot is shown for each condition. The estimated half-lives are (number of repeats in parenthesis): Sc[m3p] 75±4 min (n = 4), Sc[m3p]/Da 230±130 min (n = 2).
