Figure 2.
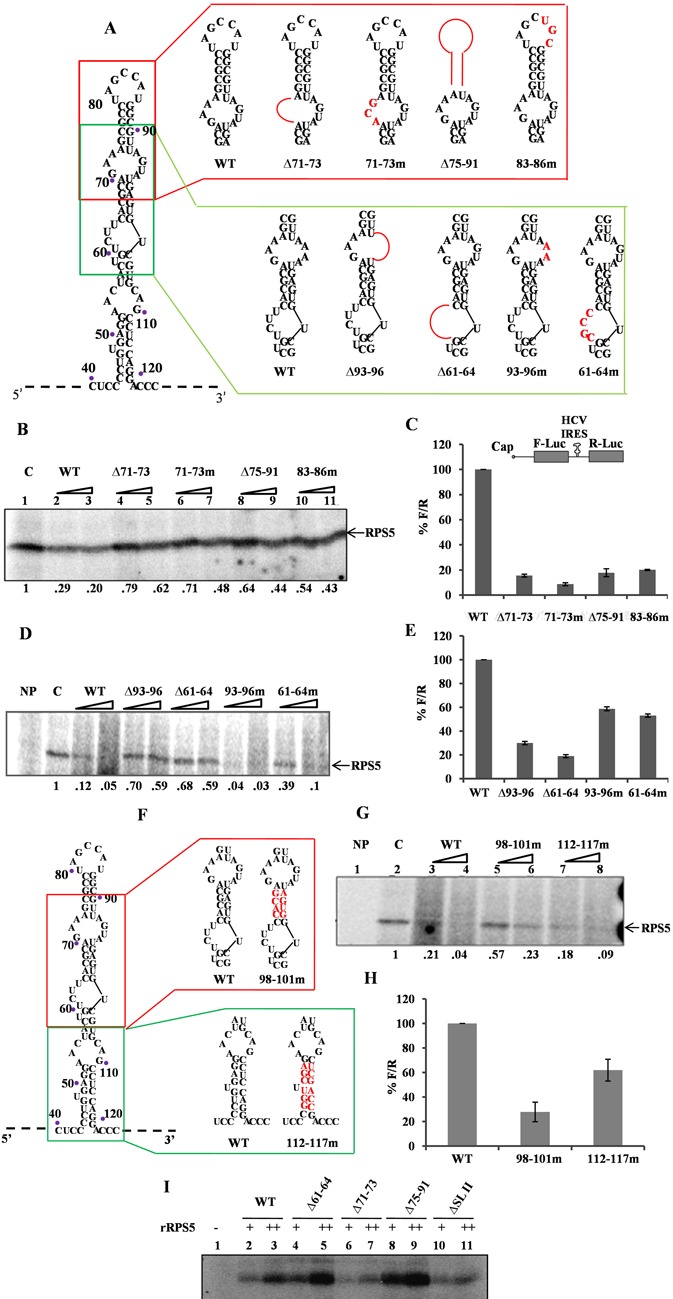
Characterization of RNA elements within HCV IRES domain II important for HCV IRES–RPS5 interaction. (A) Schematic representation of HCV IRES domain II wild type and mutant constructs used in the study. Mutated regions are shown in red. (B and D) Competition UV cross-linking of 40S ribosomal subunit with wild-type HCV IRES in presence of either unlabelled wild-type HCV IRES or domain II mutant IRES RNAs. This is a representative image of three independent experiments. (C and E) In vitro translation of wild type and mutant HCV bicistronic RNAs using rabbit reticulocyte lysate (RRL). Graph represents percentage luciferase activity (% F/R) for wild type and mutant bicistronic RNAs. Graph represents the average of three independent experiments. (F) Schematic representation of HCV IRES domain II wild type and mutant constructs used in the study. Mutated regions are shown in red. (G) Competition UV cross-linking of 40S ribosomal subunit with wild type HCV IRES in presence of either unlabelled wild type HCV IRES or domain II mutant IRES RNAs. This is a representative image of three independent experiments. (H) In vitro translation of wild type and mutant HCV bicistronic RNAs using rabbit reticulocyte lysate (RRL). Graph represents the average of three independent experiments. (I) UV cross-linking of 32P wild type and mutant HCV IRES RNAs (100 pmol) with increasing concentration (2 and 4 pmol) of recombinant RPS5. This is a representative image of three independent experiments.
