Figure 7.
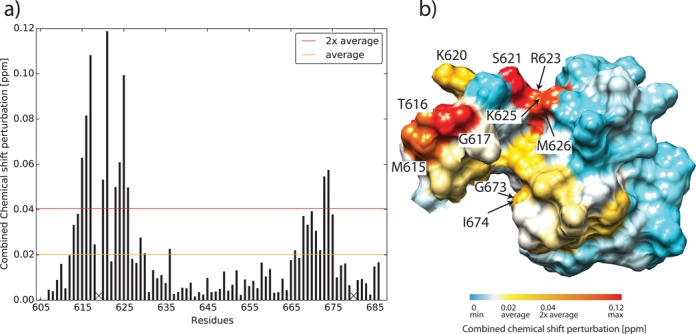
(a) Combined 1H and 15N chemical shift perturbation (CSP) of the Sso MCM C-terminal WH domain. [1H,15N]-TROSY-HSQC spectra of 15N labeled Sso MCM C-terminal WH domain and Sso MCMΔN-term (ILI555DSD) were recorded (Supplementary Figure S19). In order to deduce differences between the isolated Sso MCM C-terminal WH domain and the same domain covalently linked to the AAA+ domain, the combined 1H and 15N chemical shifts of the Sso MCM C-terminal WH domain were compared. 1H/15N scaling factors for glycine and non-glycine residues were 1/5 and 1/8, respectively (45). Proline residues without observable amide resonance are denoted with X. (b) CSP mapping onto the surface representation of Sso MCM C-terminal domain, residues E605-D610 experiencing a CSP<CSPaverage are omitted for clarity. Residues for which CSP>CSPaverage are color-coded ranging from yellow to red. Residues for which CSP>CSP2xaverage are labeled with residue numbers.
