Abstract
BACKGROUND
Recent evidence suggests that insufficient oxidative capacity or mitochondrial dysfunction may play a causal role in the development of high blood pressure. However, this hypothesis has not been tested in the general population. We hypothesized that lactate, a measure of oxidative capacity, would be positively associated with incident hypertension even after accounting for traditional hypertension risk factors.
METHODS
Plasma lactate was measured in 5,554 participants from the Atherosclerosis Risk in Communities (ARIC) Study with no subclinical or diagnosed hypertension at baseline (1996–1998). Incident hypertension was defined by self-report or hypertension medication use. Analyses were performed with Cox proportional hazards models.
RESULTS
The mean age was 61.9 years, and the mean lactate was 0.8 mmol/L. During a median follow-up period of 11.9 years (range = 26.9 days to 13.4 years), there were 3,849 new cases of hypertension. The fourth quartile of lactate (compared with the first quartile) was associated with an elevated risk of hypertension (hazard ratio (HR) = 1.18; 95% confidence interval (CI) = 1.07–1.31) even after adjustment for traditional risk factors, including baseline systolic and diastolic blood pressure. This association was stronger when the population was restricted to participants with normal blood pressure (<120mm Hg/<80mm Hg; HR = 1.42; 95% CI = 1.23–1.63). In strata of sex, the association was strong in women vs. null in men (P interaction = 0.01).
CONCLUSIONS
Plasma lactate is associated with incident hypertension in women, especially with a normal blood pressure (<120mm Hg/<80mm Hg). Future studies should elucidate the mechanisms underlying these observations.
Keywords: ARIC, blood pressure, cohort, hypertension, lactate.
Hypertension is the most common diagnosis in US ambulatory visits1,2 and a leading cause of stroke, heart disease, and kidney failure. Despite its high prevalence and significant consequences, the pathogenesis of hypertension remains incompletely understood. Blood lactate, a product of anaerobic respiration, is a marker of insufficient oxidative capacity; when oxidative capacity decreases, blood lactate rises as a consequence of increased flux through anaerobic pathways.3,4 Previous studies suggest that lactate is elevated among individuals with insulin resistance and obesity.5,6 Moreover, a small number of clinical studies have shown that lactate is associated with hypertension.3,7,8 However, these studies were mainly cross-sectional7,8 or limited to obese individuals.3 Whether elevations in lactate precede a diagnosis of hypertension is unknown.
The purpose of this study was to (i) describe the relationship between lactate and incident hypertension after accounting for traditional hypertension risk factors, including baseline systolic and diastolic blood pressure (BP), and (ii) to evaluate whether this association was modified by other hypertension risk factors. We hypothesized that lactate would be independently associated with incident hypertension, reflecting underlying decreased oxidative capacity, before the diagnosis of hypertension.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based, prospective cohort of 15,792 adults. Enrollment of predominantly black and white adults aged 45–64 years occurred from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland) in the period from 1987 to 1989. The fourth scheduled study visit (1996–1998) was attended by 11,656 participants and consisted of physical examinations, medical interviews, and laboratory tests. The fourth visit served as the baseline for our prospective study because lactate was measured in stored plasma collected during this visit.
Participants with the following characteristics were excluded from our study population: (i) prevalent cases of diagnosed or subclinical hypertension (n = 5,557); (ii) not fasting ≥8 hours (n = 604); (iii) missing valid measurements of plasma lactate (n = 170); (iv) not contributing follow-up time (n = 234); (v) underrepresented racial groups (n = 31); or (vi) missing relevant covariable data (n = 499). The total number of excluded participants was 6,102 (note that some participants were excluded for multiple reasons), resulting in a study population of 5,554. Prevalent cases of diagnosed or subclinical hypertension were defined as (i) a mean systolic BP ≥140mm Hg (based on 2 measurements),9 (ii) a mean diastolic BP ≥90mm Hg (based on 2 measurements),9 or (iii) hypertension medication use during the past 2 weeks.
The study protocol was approved by institutional review boards at each of the four clinical sites, and written informed consent was obtained from all participants.
Plasma lactate
Plasma specimens were collected during visit 4 in 1996–1998 and stored at −70 ºC in sodium fluoride–potassium oxalate tubes. Specimens were kept on ice and plasma and were measured within 15 minutes of collection to avoid hemolysis. In 2011, lactate was measured using an Olympus AU400e auto-analyzer (Olympus, Hamburg, Germany) by an enzymatic reaction that converts lactate to pyruvate.10 The inter-assay coefficient of variation was 4.4% (based on the average of 3 laboratory plasma control pools with means 8.9, 11.5, and 36.0mg/dl).
Incident hypertension
Among persons without hypertension at baseline (after excluding the prevalent cases as described above), we identified incident cases of hypertension between baseline (ARIC visit 4, 1996–1998) and 12 August 2012 (the last follow-up date available) during annual telephone calls to all participants. Throughout this study, response rates for annual telephone follow-up averaged >90%. Participants were classified as incident cases if they reported a physician diagnosis of hypertension or hypertension medication use. The date a participant reported hypertension served as the date of diagnosis. The participants who did not develop hypertension were administratively censored on the date of their last annual follow-up response.
Covariables
Data collection was carried out by highly trained personnel using standardized protocols with comprehensive quality control measures previously described.11,12 Age, sex, race, and smoking status were assessed in 1996–1998 by self-report. Leisure time, an index of physical activity, was assessed during the first visit of ARIC (1987–1989) using a modified Baeke Physical Activity questionnaire.13 Prevalent coronary heart disease was based on a prevalent coronary heart disease at visit 1 (determined based on electrocardiograms, self-report, or previous coronary procedures) or an adjudicated event developed between visit 1 and visit 4. Diabetes status was defined based on a fasting glucose ≥126mg/dl, a self-reported diagnosis of diabetes, or self-reported diabetes medication use. Waist circumference was measured at the umbilical level as well as standing height and weight to calculate body mass index. High-density lipoprotein cholesterol, triglycerides, fasting glucose, and fasting insulin were measured in serum. Triglycerides were log10 transformed to normalize distribution. Fasting glucose was quantified using a hexokinase method.
At each visit, BP was measured early during the clinic visit after 5 minutes of rest and before most procedures (e.g, physical examination and phlebotomy). Certified technicians used a random-zero sphygmomanometer to take 3 BP measurements for the first 3 visits, recording the average of the second and third measurements, and 2 BP measurements for the fourth visit and the Carotid Magnetic Resonance Imaging (CARMRI) substudy, which were averaged. The BP measurements from CARMRI were used for the sensitivity analysis.
Statistical analysis
Baseline characteristics of the study population were expressed as means and proportions by quartiles of lactate. Trends across quartiles were evaluated using the median of each quartile as a continuous variable. Cox proportional hazards models were used to examine the association between baseline plasma lactate (divided into quartiles or as a continuous variable) and risk of incident hypertension using 3 nested models. This was performed in the population overall and by sex. Model 1 was adjusted for age, sex, race-study center (a composite variable including both race and ARIC study center). Model 2 was adjusted for all variables in model 1 plus body mass index and waist circumference. Model 3 was adjusted for all variables in model 2 plus baseline systolic BP, baseline diastolic BP, high-density lipoprotein cholesterol, log10 triglycerides, prevalent coronary heart disease, smoking status (never, former, or current), fasting glucose, insulin, diabetes status, and physical activity index. We used a restricted cubic spline to depict the continuous relationship between plasma lactate and hypertension adjusted for model 3 variables. In this model, the 25th percentile served as the reference lactate concentration, and knots were placed at the 25th, 50th, and 75th percentiles. This was further overlaid with a kernel density plot to characterize the distribution of baseline lactate concentrations. In sensitivity analyses we also adjusted for low-density lipoprotein cholesterol, estimated glomerular filtration rate, log10-transformed high-sensitivity C-reactive protein, uric acid concentration, education level, and alcohol use. These variables had little influence on the association of lactate with incident hypertension and, therefore, were not included in our final models.
We also examined the association of lactate with incident hypertension by strata of traditional hypertension risk factors that were also associated with lactate. These factors were sex, race (black vs. white), obesity (no or yes; ≥30kg/m2), prehypertension (no or yes; systolic BP ≥120mm Hg and <140mm Hg or diastolic BP ≥80mm Hg and <90mm Hg9), and diabetes (no or yes; fasting glucose ≥126mg/dl, a self-reported diagnosis of diabetes, or self-reported diabetes medication use).
We also performed the following sensitivity analyses: (i) restriction of the study population to participants with a plasma lactate <2 mmol/L, corresponding to the clinical value of mild hyperlactemia;14 (ii) restriction of the study population to participants without prevalent coronary heart disease because of its known association with lactate; (iii) restriction of the study population to participants without prehypertension (i.e., normal BP, systolic BP <120mm Hg and a diastolic BP <80mm Hg9). These were performed using models 1–3. Using model 3 only, we also compared the prospective association of lactate (per 1 SD) and incident hypertension with baseline systolic and diastolic BP (per 1 SD) in the overall population, as well as in subpopulations restricted to either a plasma lactate <2 mmol/L or participants without coronary heart disease. We conducted this comparison in strata of normal BP and prehypertension as well as by sex. Finally, to address potential misclassification due to self-reported hypertension, we repeated our prospective analysis in 1,153 ARIC participants who participated in CARMRI conducted in 2005–2006.15 For this analysis, we limited incident cases of hypertension to CARMRI participants who were using BP-lowering medications or who were hypertensive by BP measurement (average of 3 systolic BP measurements ≥140mm Hg or 3 diastolic BP measurements ≥90mm Hg).
RESULTS
In our study population of 5,554 participants, the mean plasma lactate was 0.8 mmol/L (SD = 0.3), ranging from 0.3 to 6.2 mmol/L (Table 1). Ninety-nine percent of the study population (n = 5,513) had a lactate concentration <2 mmol/L, well within the normal range of lactate.14 Age, smoking status, and physical activity index did not differ significantly across quartiles of baseline lactate. Male sex, black race, prehypertension status, systolic BP, diastolic BP, prevalent coronary disease, prevalent diabetes, waist circumference, triglycerides, fasting glucose, and fasting insulin demonstrated positive trends across lactate quartiles (all P trends < 0.001). In contrast, high-density lipoprotein cholesterol demonstrated a monotonic decrease across increasing lactate quartiles (P trend < 0.001).
Table 1.
Baseline (visit 4) characteristics of Atherosclerosis Risk in Communities participants by plasma lactate level
| Overall (n = 5,554) | Quartile 1: 0.27–0.56 (n = 1,404) | Quartile 2: 0.57–0.69 (n = 1,394) | Quartile 3: 0.70–0.88 (n = 1,372) | Quartile 4: 0.89–6.16 (n = 1,384) | P value for trenda | |
|---|---|---|---|---|---|---|
| Plasma lactate, mmol/Lb | 0.8 (0.3) | 0.5 (0.1) | 0.6 (0.0) | 0.8 (0.1) | 1.2 (0.3) | — |
| Age, y, mean (SD) | 61.9 (5.5) | 61.6 (5.5) | 62.3 (5.5) | 62.0 (5.5) | 61.9 (5.6) | 0.79 |
| Men, no. (%) | 2,503 (45.1) | 503 (35.8) | 660 (47.3) | 721 (52.6) | 619 (44.7) | <0.001 |
| Black, no. (%) | 713 (12.8) | 118 (8.4) | 146 (10.5) | 178 (13.0) | 271 (19.6) | <0.001 |
| Prehypertension, no. (%)c | 2,590 (46.6) | 594 (42.3) | 640 (45.9) | 659 (48.0) | 697 (50.4) | <0.001 |
| Systolic blood pressure, mm Hg, mean (SD) | 117.4 (11.8) | 116.0 (12.2) | 117.1 (11.8) | 117.9 (11.6) | 118.8 (11.5) | <0.001 |
| Diastolic blood pressure, mm Hg, mean (SD) | 68.1 (8.6) | 67.5 (8.4) | 67.7 (8.7) | 68.5 (8.5) | 68.5 (8.7) | <0.001 |
| Prevalent coronary disease, no. (%) | 316 (5.7) | 60 (4.3) | 68 (4.9) | 81 (5.9) | 107 (7.7) | <0.001 |
| Prevalent diabetes, no. (%) | 526 (9.5) | 45 (3.2) | 83 (6.0) | 112 (8.2) | 286 (20.7) | <0.001 |
| Smoking status, no. (%) | ||||||
| Never | 2,265 (40.8) | 589 (42.0) | 574 (41.2) | 553 (40.3) | 549 (39.7) | 0.18 |
| Former | 2,431 (43.8) | 598 (42.6) | 597 (42.8) | 603 (44.0) | 633 (45.7) | 0.06 |
| Current | 858 (15.4) | 217 (15.5) | 223 (16.0) | 216 (15.7) | 202 (14.6) | 0.44 |
| Body mass index, kg/m2, mean (SD) | 27.6 (5.0) | 26.4 (4.5) | 27.1 (4.8) | 28.1 (4.9) | 29.0 (5.2) | <0.001 |
| Waist circumference, cm, mean (SD) | 99.0 (13.4) | 95.4 (13.0) | 97.6 (12.9) | 100.5 (13.2) | 102.5 (13.5) | <0.001 |
| Physical activity index, units, mean (SD)d | 2.3 (0.6) | 2.3 (0.6) | 2.4 (0.6) | 2.3 (0.6) | 2.3 (0.6) | 0.14 |
| Triglycerides, mg/dl, median (interquartile range)e | 117 (85–165) | 97 (73–129) | 108 (81–145) | 128 (93–174) | 154 (104–221) | <0.001 |
| High-density lipoprotein cholesterol, mg/dl, mean (SD) | 50.8 (16.7) | 55.1 (17.0) | 51.9 (17.2) | 48.8 (15.7) | 47.5 (15.6) | <0.001 |
| Fasting glucose, mg/dl, mean (SD) | 104.1 (27.3) | 96.7 (13.6) | 100.8 (20.5) | 103.9 (22.6) | 115.0 (41.1) | <0.001 |
| Fasting insulin, μm/ml, mean (SD) | 11.3 (8.8) | 9.1 (6.2) | 10.0 (6.7) | 11.9 (10.6) | 14.3 (9.9) | <0.001 |
| Statin use, no. (%) | 467 (8.4) | 98 (7.0) | 101 (7.2) | 119 (8.7) | 149 (10.8) | <0.001 |
| Loop diuretic use, no. (%) | 98 (1.8) | 10 (0.7) | 24 (1.7) | 19 (1.4) | 45 (3.3) | <0.001 |
| Nonsteroidal anti-inflammatory drug use, no. (%) | 1,502 (27.0) | 393 (28.0) | 408 (29.3) | 351 (25.6) | 350 (25.3) | 0.03 |
a P value for trend evaluated with linear or logistic regression using the median lactate value for each quartile as a continuous variable.
bLactate mmol/L may be converted to mg/dl by dividing by 0.111
cPrehypertension defined as a systolic blood pressure of 120–139mm Hg or a diastolic blood pressure of 80–89mm Hg.
dPhysical activity index was assessed at Atherosclerosis Risk in Communities visit 1.
ePresented as the median (interquartile range). To evaluate trend, this variable was log10 transformed to normalize its distribution.
Over a median follow-up period of 11.9 years (range = 26.9 days to 13.4 years), there were 3,849 new cases of self-reported diagnosed hypertension. The unadjusted 10-year cumulative incidence across quartiles of lactate concentration was 14.6%, 18.3%, 21.5%, and 32.3% (quartiles 1–4, respectively) (Supplementary Figure S1). There was a significant association between quartiles of lactate and risk of incident hypertension after adjustment for demographic characteristics (model 1; P trend = 0.001) (Table 2). This trend changed minimally with adjustment for measures of adiposity (model 2; P trend = 0.001). The association remained significant, although moderately attenuated, after further adjustment for systolic and diastolic BP as well as other hypertension risk factors (model 3; P trend = 0.001). Compared with the first quartile of lactate, the highest quartile was associated with a significant increase in risk of hypertension (hazard ratio (HR) = 1.18; 95% confidence interval (CI) = 1.07–1.31), even after adjustment for all covariables, including baseline BP. Similarly, when examined as a continuous variable with adjustment for all covariables, every 1 mmol/L higher baseline lactate was associated with 1.22 (95% CI = 1.09–1.36) times the hazard of incident hypertension (model 3). When examined by strata of sex, we found that the relationship between lactate and incident hypertension was stronger in women and no longer significant in men. These patterns of associated were reflected in our restricted cubic spline models (Figure 1a,b). Overall and in women, we observed a positive linear relationship between baseline lactate concentration and subsequent risk of diagnosed hypertension with no evidence of a threshold effect. However, among men, lactate was not associated with incident hypertension.
Table 2.
Hazard ratios (95% confidence intervals) for developing incident hypertension by categorical and continuous lactate concentration by sex and overall
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Women (n = 3,051) | |||
| Lactate quartiles, mmol/L | |||
| 0.27–0.56 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 0.57–0.69 | 1.09 (0.97–1.23) | 1.08 (0.96–1.21) | 1.02 (0.91–1.15) |
| 0.70–0.88 | 1.12 (0.99–1.27) | 1.10 (0.97–1.24) | 1.03 (0.90–1.17) |
| 0.89–6.16 | 1.63 (1.45–1.84) | 1.59 (1.41–1.80) | 1.37 (1.20–1.56) |
| P trend a | <0.001 | <0.001 | <0.001 |
| Per 1 mmol/L of lactate | 1.74 (1.54, 1.97) | 1.70 (1.50–1.93) | 1.43 (1.24–1.66) |
| P value | <0.001 | <0.001 | <0.001 |
| Men (n = 2,503) | |||
| Lactate quartiles, mmol/L | |||
| 0.3–0.6 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 0.6–0.7 | 1.08 (0.94–1.24) | 1.09 (0.95–1.26) | 0.98 (0.86–1.13) |
| 0.7–0.9 | 1.05 (0.92–1.20) | 1.05 (0.91–1.20) | 0.95 (0.83–1.09) |
| 0.9–6.2 | 1.30 (1.13–1.50) | 1.26 (1.09–1.45) | 0.97 (0.83–1.13) |
| P trend a | <0.001 | 0.003 | 0.67 |
| Per 1 mmol/L of lactate | 1.38 (1.20, 1.59) | 1.34 (1.15–1.55) | 0.97 (0.81–1.16) |
| P value | <0.001 | <0.001 | 0.72 |
| Overall (n = 5,554) | |||
| Lactate quartiles, mmol/L | |||
| 0.3–0.6 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 0.6–0.7 | 1.09 (1.00–1.19) | 1.08 (0.99–1.19) | 1.01 (0.92–1.11) |
| 0.7–0.9 | 1.09 (0.99–1.19) | 1.07 (0.98–1.18) | 1.00 (0.91–1.10) |
| 0.9–6.2 | 1.47 (1.34–1.61) | 1.44 (1.31–1.58) | 1.18 (1.07–1.31) |
| P trend a | <0.001 | <0.001 | 0.001 |
| Per 1 mmol/L of lactate | 1.56 (1.43, 1.71) | 1.53 (1.39–1.67) | 1.22 (1.09–1.36) |
| P value | <0.001 | <0.001 | 0.001 |
The interaction between sexes was significant (see Figure 2 for details). Model 1 included age, sex, and race–Atherosclerosis Risk in Communities center (a composite variable of both race and ARIC study center). Model 2 included all of the variables in model 1 plus body mass index and waist circumference. Model 3 included all of the variables in model 2 plus systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, log10 triglycerides, prevalent coronary disease, smoking status, fasting glucose, insulin, diabetes status, and physical activity index.
a P value for trend evaluated using a continuous variable based on the median lactate in each quartile.
Figure 1.
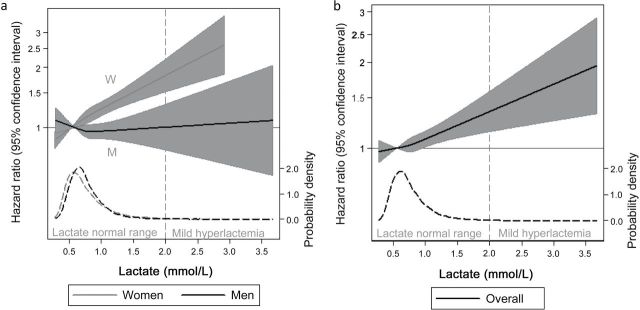
Adjusted hazard ratios (solid line) for incident self-reported hypertension between Atherosclerosis Risk in Communities (ARIC) visit 4 and 12 August 2012, according to baseline concentrations of plasma lactate values from a restricted cubic spline model. (a) Stratified by sex. (b) Overall population. Shaded region represents the 95% confidence intervals. A vertical dashed line (grade) represents the cutpoint for mild hyperlactemia. This model was expressed relative to the 25th percentile of lactate with knots specified at the 25th, 50th, and 75th percentiles and was adjusted for age, sex, race-center (a composite variable of both race and ARIC study center), body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, log10 triglycerides, prevalent coronary heart disease, smoking status (never, former, or current), fasting glucose insulin, diabetes status, and physical activity index. The plot was truncated at the 0.5th and 99.5th percentiles of lactate. The hazard ratios are shown on a natural log scale. This figure is overlaid with a kernel density plot (dashed line; gray represents women, black represents men), showing the overall distribution of baseline lactate values.
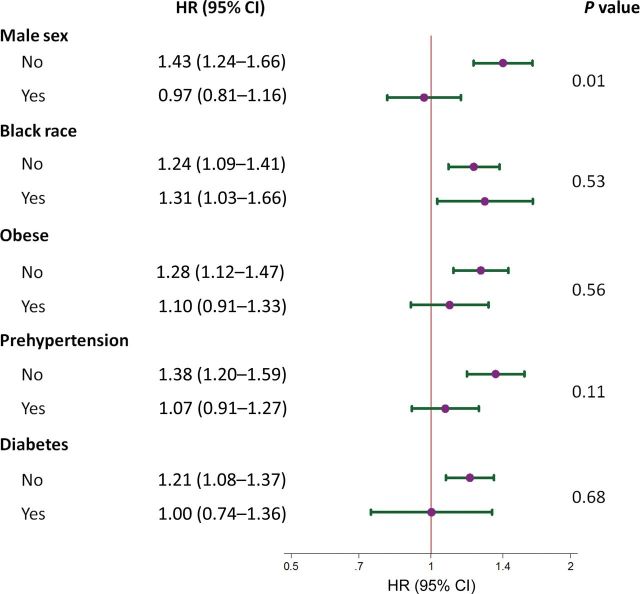
Effect modification by factors associated with hypertension was examined in Figure 2. There was no evidence of effect modification by race, obesity, prehypertension, or diabetes status. In contrast, the association of lactate with incident hypertension was significantly different among women compared with men even after full adjustment (P interaction = 0.01). Although lactate was strongly associated with the risk of hypertension in women (HR = 1.43; 95% CI = 1.24–1.66), lactate was not associated with the risk of hypertension in men (HR = 0.97; 95% CI = 0.81–1.16).
Figure 2.
Forest plot portraying the hazard ratio (HR) and 95% confidence interval (CI) of the association between lactate (per 1 mmol/L) and incident hypertension. Strata were sex (female or male), race (white or black), obese (<30kg/m2 or ≥30kg/m2), prehypertension (no or yes; systolic blood pressure ≥120mm Hg and <140mm Hg or diastolic blood pressure ≥80mm Hg and <90mm Hg), or diabetes (no or yes; fasting glucose ≥126mg/dl, a self-reported diagnosis of diabetes, or self-reported diabetes medication use). In general, all models were adjusted for age, sex, race–Atherosclerosis Risk in Communities (ARIC) center (a composite variable including both race and ARIC study center), high-density lipoprotein cholesterol, log10 triglycerides, prevalent coronary disease, smoking status, body mass index, waist circumference, fasting glucose, insulin, diabetes status, and physical activity index. When evaluating an interaction between strata of race, race-center variable (a composite variable including both race and ARIC study center) was replaced with a dichotomous black (yes/no) covariable. In strata of obesity, models were not adjusted for the body mass index covariable. Strata of prehypertension did not include adjustment for systolic or diastolic blood pressure. P values comparing strata were determined using interaction terms.
The observed associations were stronger when the study population was restricted to participants in the normal range of lactate (Supplementary Table S1). Excluding participants with prevalent coronary heart disease had virtually no effect on our findings (Supplementary Table S2). However, restricting our population to participants with normal systolic BP (<120mm Hg) and diastolic BP (<80mm Hg) strengthened both the magnitude and significance of our findings (Supplementary Table S3). In our comparison of the prospective association between lactate and incident hypertension with baseline systolic and diastolic BP, we found lactate to be more significantly associated with incident hypertension than baseline systolic and diastolic BP, particularly among women with a normal BP at baseline (Supplementary Tables S4 and S5). Furthermore, expanding our fully adjusted model to include low-density lipoprotein cholesterol, estimated glomerular filtration rate, log10 high-sensitivity C-reactive protein, uric acid concentration, education level, and alcohol use did not significantly change our findings (Supplementary Table S6). Finally, in our CARMRI sensitivity analysis we found that, although our findings were attenuated with a smaller sample size, the pattern of association was preserved. In particular, lactate (per 1 mmol/L lactate increase) was still associated with incident hypertension, both overall (HR = 1.62; 95% CI = 1.10–2.39) and in strata of women (HR = 2.33; 95% CI = 1.45–3.73) (Supplementary Table S7).
DISCUSSION
This study represents the first population-based, prospective cohort study of the association between plasma lactate and incident hypertension. Plasma lactate was associated with risk of incident hypertension among women over more than a decade of follow-up. This association was independent of traditional hypertension risk factors, including baseline BP. Furthermore, lactate was more strongly associated with incident hypertension when restricted to a population with normal systolic and diastolic BP, suggesting that elevations in lactate preceded elevations in BP.
Our study contributes to existing literature evaluating the association between lactate concentrations and hypertension. Several studies have reported a positive association between lactate and BP. One early cross-sectional study of 19 lean adults (16% women) showed that arterial lactate concentrations were associated with mean arterial pressure (β from linear regression = 0.55; P = 0.02).7 Another study of 55 adults (9% women) with varying degrees of obesity and insulin resistance, found lactate to be strongly associated with systolic BP (correlation coefficient = 0.69; P < 0.001) and diastolic BP (correlation coefficient = 0.59; P < 0.005).8 More recently, a weight-loss study of 40 obese adults with the metabolic syndrome found that change in lactate was positively associated with change in diastolic BP (P = 0.02) and mean arterial pressure (P = 0.05), but not systolic BP (P = 0.36), after adjustment for baseline lactate, change in body mass index, age, and sex.3 Not all studies support a positive relationship between lactate and BP, however. One cross-sectional study (n = 44; 50% women) reported that lactate measured in serum (rather than plasma) was not associated with BP.16 The reasons for these null findings are unclear and could be due to a variety of factors. The sample handling procedures in the study were not described, and it is unclear whether the specimens were processed in an optimal fashion to avoid potential instability of the lactate levels.17,18 In addition, owing to the small number of patients studied, the null findings could have been due to a lack of statistical power. Contrary to the above, our study found plasma lactate to be cross-sectionally associated with baseline systolic and diastolic BP. Furthermore, lactate was prospectively associated with incident hypertension independent of baseline BP measurements and in a population with normal BP (systolic BP <120mm Hg and diastolic BP <80mm Hg).
Lactate is an indicator of decreased oxidative capacity. Other conditions involving decreased oxidative capacity are also associated with BP, including low fitness,19–23 a state that involves reduced capillary density,24 and increased adiposity,25 a state in which tissue mass outpaces vessel growth.26,27 Furthermore, emerging evidence suggests that inadequate angiogenesis, which creates a disparity in blood supply and tissue oxygen demand, may play an important role in the development of hypertension. Recent genome-wide association studies have linked angiogenic genes with hypertension.28 Mechanistic studies have shown that angiogenic promoters reduce BP and angiogenic inhibitors increase BP.29,30 Furthermore, microvasculature damage is also associated with BP,31 with reduced microvascular density and capillary rarefaction being present before32 and in early hypertension.33,34 We speculate that the association between lactate and incident hypertension is representative of insufficient angiogenesis leading to decreased oxidative capacity and subsequent high BP. Alternatively, decreased oxidative capacity may be a marker of insufficient vascular capacity, the proximal cause of high BP in this scenario, and not a mediator in the pathway leading to high BP. Examination of these hypotheses is beyond the scope of this study, however.
We found a significant interaction by sex with regard to the association between lactate and incident hypertension. Whereas lactate was significantly associated with incident hypertension in women, it was not associated with incident hypertension in men. This observation could be due to survival bias, in that there is evidence that men develop hypertension earlier than women.35 Because our study population was conducted in an older population (mean age = 62 years), it is possible that many of the men who were going to develop hypertension during their lifetime had done so before the start of this study and were thus excluded at baseline. There is also evidence that age is more significantly associated with an increase in BP in women compared with men.36 As a result, the men included in our study may have been less prone to develop hypertension, lowering our power to observe an effect. Equally possible is that this interaction represents a physiologic effect of sex. Lactate concentrations are higher in men vs. women. Further, there is evidence that lactate reflects exercise capacity differently in elderly men and women,37 which might be related to menopause.38 Finally, it is also possible that other factors are more important for the development of hypertension among men compared with women, causing the lactate association in men to be nonsignificant. We are unable to differentiate between the methodological and physiologic explanations for the sex interaction observed in this study, however.
There are several limitations to this study that warrant discussion. We only had a single measurement of plasma lactate, which is less precise than multiple measurements. We attempted to minimize this imprecision by requiring the population to be both fasting and resting during the time of blood draw; however, it is possible that imprecision in lactate measures attenuated our results. Furthermore, incident hypertension was based on self-report rather than measurements of systolic or diastolic BP. This could result in misclassification or missed cases of incident hypertension, attenuating our findings. However, previous reports have suggested that self-reported disease status can be both a valid and specific measure in population-based studies.39,40 Finally, residual confounding is always a concern with observational studies. For example we were not able to adjust for family history of hypertension, sodium intake, or stress score in this study.
Our study has a number of strengths as well. Our study was performed in a well-designed, prospective study with a large, diverse sample size and long-term follow-up. Furthermore, the study involved rigorous assessment of laboratory markers and anthropometric measures associated with hypertension, improving our ability to address confounding.
In conclusion, we found that baseline elevations in plasma lactate were associated with an increased risk of incident hypertension in women with normal BP independently of baseline BP and other known hypertension risk factors. Future studies are necessary to better understand the mechanism behind the association between lactate and incident hypertension as well as to confirm the sex interaction observed in our study.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by P60 Diabetes P&C Core of the Diabetes Research Training Center (5P60DK079637-04). Dr Young and Dr Selvin were supported by NIH/NHLBI RO1DK085458 grant. S.P.J. and J.K.B. were supported by NIH/NHLBI T32HL007024 Cardiovascular Epidemiology Training Grant. The Atherosclerosis Risk in Communities Study is carried out as a collaborative work supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268 201100007C, HHSN268201100008C, HHSN2682011000 09C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). We thank the staff and participants of the ARIC Study for their important contributions. Parts of this work were presented at the High Blood Pressure Research 2013 Scientific Sessions, New Orleans, Louisiana, September 11−14, 2013.
REFERENCES
- 1. Centers for Disease Control. National Ambulatory Medical Care Survey: 2008. http://www.cdc.gov/nchs/fastats/docvisit.htm.
- 2. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 3. Crawford S O, Ambrose MS, Hoogeveen RC, Brancati FL, Ballantyne CM, Young JH. Association of lactate with blood pressure before and after rapid weight loss. Am J Hypertens 2008; 21:1337–1342. [DOI] [PubMed] [Google Scholar]
- 4. Zagari F, Jordan M, Stettler M, Broly H, Wurm FM. Lactate metabolism shift in CHO cell culture: the role of mitochondrial oxidative activity. N Biotechnol 2013; 30:238–245. [DOI] [PubMed] [Google Scholar]
- 5. DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J 1992; 6:2405–2412. [DOI] [PubMed] [Google Scholar]
- 6. Doar JW, Wynn V, Cramp DG. Blood pyruvate and plasma glucose levels during oral and intravenous glucose tolerance tests in obese and non-obese women. Metab Clin Exp 1968; 17:690–701. [DOI] [PubMed] [Google Scholar]
- 7. Baron AD, Brechtel-Hook G, Johnson A, Hardin D. Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension 1993; 21:129–135. [DOI] [PubMed] [Google Scholar]
- 8. Jansson PA, Larsson A, Lönnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1998; 28:813–818. [DOI] [PubMed] [Google Scholar]
- 9. National Heart Lung and Blood Institute. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure - Complete Report. http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. [PubMed] [Google Scholar]
- 10. Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972; 97:142–145. [DOI] [PubMed] [Google Scholar]
- 11. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003; 52:1799–1805. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999; 353:1649–1652. [DOI] [PubMed] [Google Scholar]
- 13. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36:936–942. [DOI] [PubMed] [Google Scholar]
- 14. Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992; 20:80–93. [DOI] [PubMed] [Google Scholar]
- 15. Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, Ballantyne C, Sharrett R, Boerwinkle E. Correlates of carotid plaque presence and composition as measured by MRI: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging 2009; 2:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iannello S, Campione R, Belfiore F. Response of insulin, glucagon, lactate, and nonesterified fatty acids to glucose in visceral obesity with and without NIDDM: relationship to hypertension. Mol Genet Metab 1998; 63:214–223. [DOI] [PubMed] [Google Scholar]
- 17. Astles R, Williams CP, Sedor F. Stability of plasma lactate in vitro in the presence of antiglycolytic agents. Clin Chem 1994; 40:1327–1330. [PubMed] [Google Scholar]
- 18. Dubé MP, Kitch DW, Parker RA, Alston-Smith BL, Mulligan K. The effect of long-term storage on measured plasma lactate concentrations and prospective lactate results from a multicenter trial of antiretroviral therapy. Clin Chem Lab Med 2005; 43:947–952. [DOI] [PubMed] [Google Scholar]
- 19. Praet SFE, Jonkers RA, Schep G, Stehouwer CD, Kuipers H, Keizer HA, van Loon LJ. Long-standing, insulin-treated type 2 diabetes patients with complications respond well to short-term resistance and interval exercise training. Eur J Endocrinol 2008; 158:163–172. [DOI] [PubMed] [Google Scholar]
- 20. Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe AM, Barker TA, Tipton KD, Wagenmakers AJ. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol (Lond) 2013; 591:641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ. Peripheral effects of endurance training in young and old subjects. J Appl Physiol 1989; 66:2844–2849. [DOI] [PubMed] [Google Scholar]
- 22. Crawford MH. Physiologic consequences of systematic training. Cardiol Clin 1992; 10:209–218. [PubMed] [Google Scholar]
- 23. Fernandes T, Nakamuta JS, Magalhães FC, Roque FR, Lavini-Ramos C, Schettert IT, Coelho V, Krieger JE, Oliveira EM. Exercise training restores the endothelial progenitor cells number and function in hypertension: implications for angiogenesis. J Hypertens 2012; 30:2133–2143. [DOI] [PubMed] [Google Scholar]
- 24. Bassett DR., Jr Skeletal muscle characteristics: relationships to cardiovascular risk factors. Med Sci Sports Exerc 1994; 26:957–966. [PubMed] [Google Scholar]
- 25. El-Atat F, Aneja A, Mcfarlane S, Sowers J. Obesity and hypertension. Endocrinol Metab Clin North Am 2003; 32:823–854. [DOI] [PubMed] [Google Scholar]
- 26. Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 2003; 58:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56:901–911. [DOI] [PubMed] [Google Scholar]
- 28. Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet 2012; 28:397–408. [DOI] [PubMed] [Google Scholar]
- 29. Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003; 107:1359–1365. [DOI] [PubMed] [Google Scholar]
- 30. Lankhorst S, Kappers MHW, van Esch JHM, Danser AHJ, van den Meiracker AH. Mechanism of hypertension and proteinuria during angiogenesis inhibition: evolving role of endothelin-1. J Hypertens 2013; 31:444–454. [DOI] [PubMed] [Google Scholar]
- 31. Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999; 150:263–270. [DOI] [PubMed] [Google Scholar]
- 32. Struijker Boudier HA, le Noble JL, Messing MW, Huijberts MS, le Noble FA, van Essen H. The microcirculation and hypertension. J Hypertens Suppl 1992; 10:S147–156. [PubMed] [Google Scholar]
- 33. Sullivan JM, Prewitt RL, Josephs JA. Attenuation of the microcirculation in young patients with high-output borderline hypertension. Hypertension 1983; 5:844–851. [DOI] [PubMed] [Google Scholar]
- 34. Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 1997; 99:1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daugherty SL, Masoudi FA, Zeng C, Ho PM, Margolis KL, O’Connor PJ, Go AS, Magid DJ. Sex differences in cardiovascular outcomes in patients with incident hypertension. J Hypertens 2013; 31:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens 1997; 11:507–514. [DOI] [PubMed] [Google Scholar]
- 37. Lötscher F, Löffel T, Steiner R, Vogt M, Klossner S, Popp A, Lippuner K, Hoppeler H, Däpp C. Biologically relevant sex differences for fitness-related parameters in active octogenarians. Eur J Appl Physiol 2007; 99:533–540. [DOI] [PubMed] [Google Scholar]
- 38. Gigli I, Bussmann LE. Exercise and ovarian steroid hormones: their effects on mitochondrial respiration. Life Sci 2001; 68:1505–1514. [DOI] [PubMed] [Google Scholar]
- 39. Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 2012; 176:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Selem SS, Castro MA, César CLG, Marchioni DML, Fisberg RM. Validity of self-reported hypertension is inversely associated with the level of education in Brazilian individuals. Arq Bras Cardiol 2013; 100:52–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.