FIG 1 .
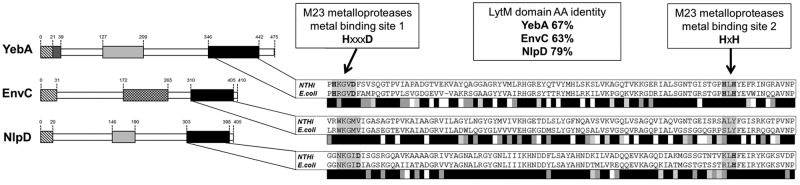
In silico analysis of NTHI LytM proteins. (Left) Domain organization of LytM proteins. LytM domains (black), LysM domains (light grey), transmembrane regions (dark grey), signal sequences (hatched boxes), and coiled coil regions (box with checkerboard pattern) are indicated. (Right) Alignments of LytM domains from NTHI and E. coli proteins are shown. Grey scale bars summarize results of LytM domain alignments of NTHI and E. coli proteins: black indicates positions that have fully conserved residues, dark grey indicates conservation between groups of strongly similar properties (scores of >0.5 in the Gonnet PAM 250 matrix), light grey indicates conservation between groups of weakly similar properties (score of <0.5 in the Gonnet PAM 250 matrix), and white indicates residues that were not conserved. AA, amino acid.
