FIG 6 .
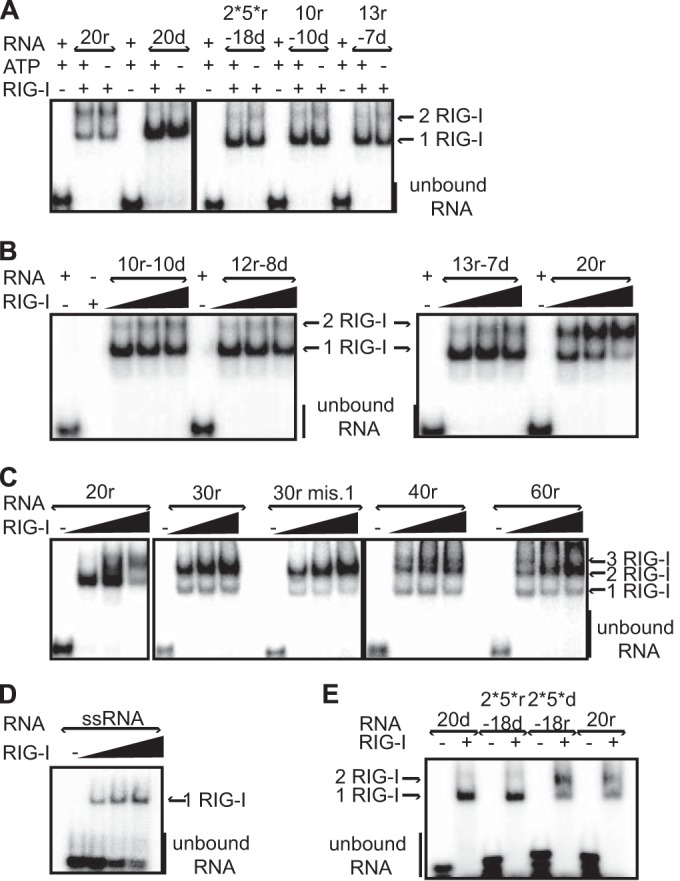
RIG-I oligomerization on RNA hybrids is RNA length dependent and ATPase independent. EMSA analysis was performed by incubating various radiolabeled RNA hybrids (250 nM) with purified his-RIG-I (2.5 μM (A and E); with increasing amount of RIG-I (4, 6, or 8 μM) in the presence of ATP and MgCl2 (B to D)). (A) RIG-I binding and oligomerization are independent of the ATPase activity. (B) dsRNA length dependence for RIG-I oligomerization. (C) The longer the dsRNA hybrid, the more easily 2-RIG-I/RNA or 3-RIG-I/RNA complexes are formed. (D) RIG-I binds ssRNA. (E) Importance of ribonucleotide content. The 2*5*d-18r molecule forms a RIG-I dimer, in contrast to its mirror molecule, 2*5*r-18d. Reactions were analyzed on native gels and revealed by phosphorimaging. See also Fig. S4 in the supplemental material.
