FIGURE 3.
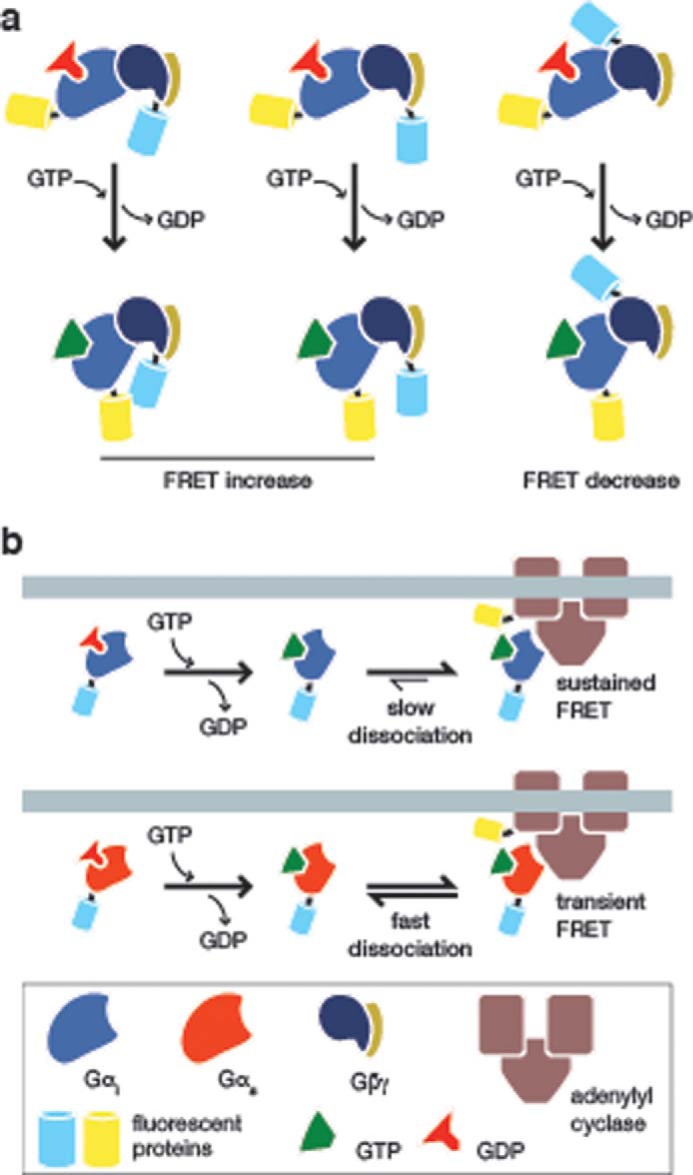
Visualizing heterotrimeric G-protein signaling. a, conformational rearrangement upon activation of the Gi heterotrimer. To test the dissociation of Gi upon activation by a GPCR, Bünemann et al. (30) generated a series of biosensors by fusing YFP to Gαi and CFP to three different positions on the Gβγ dimer. Although one of these biosensors produced a FRET decrease upon GPCR stimulation (right panel), the other two both produced increasing FRET responses (left and middle panels), suggesting that Gi proteins undergo a conformational rearrangement upon activation and do not fully dissociate, as in the case of the Gs heterotrimer. b, monitoring Gαi interaction with adenylyl cyclase. Milde et al. (35) recently examined the interaction between Gαi and adenylyl cyclase by fusing CFP to Gαi1 and YFP to AC5. The activation of Gαi1 by GPCR signaling results in its interaction with AC5, leading to a FRET increase (upper panel). However, when compared with Gαs (lower panel), Gαi1 was observed to dissociate very slowly from AC5, which the authors suggested may be a mechanism for the self-regulation of AC5 activity in cells. For clarity, Gβγ is not shown.
