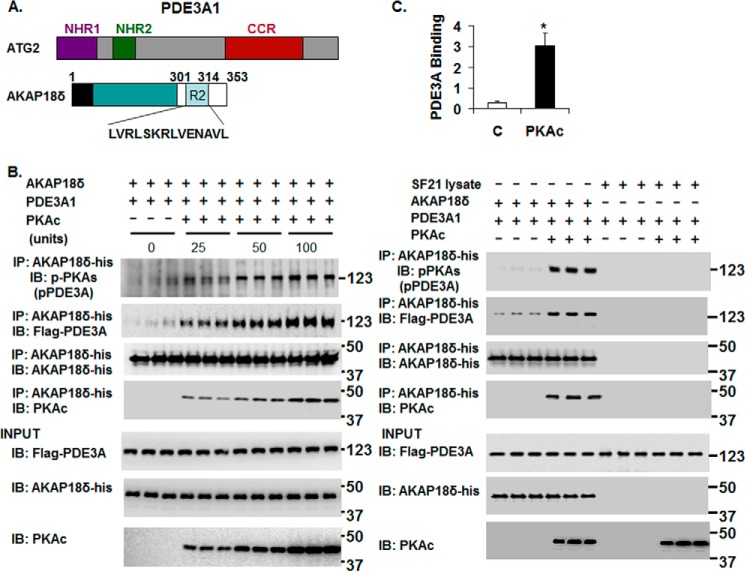
FIGURE 7.
rPKAc-induced phosphorylation of rhPDE3A increases its interaction with rat rAKAP18δ. A, schemes representing hPDE3A1 protein domains (NHR1, trans-membrane domain (obligatory membrane insertion domain); NHR2, membrane association domain; CCR, conserved C-terminal catalytic region) and rAKAP18δ protein domains, with RII binding site (aa 301–314) and a unique N terminus (aa 1–26). B and C, His-tagged rAKAP18δ (100 ng) and 50 units of FLAG-tagged rhPDE3A1 were incubated for 30 min at 30 °C in phosphorylation buffer containing 200 μm ATP and 5 mm MgCl2 in the absence or presence of the indicated concentrations (units) of rPKAc (B) or in the absence or presence of 50 units of rPKAc (C). B and C, as described under “Methods,” proteins were immunoprecipitated (IP) with anti-His mAb magnetic beads, and immunoprecipitated proteins bound to the anti-His mAb magnetic beads were eluted by boiling in Laemmli SDS buffer (200 μl). Samples (15–20 μl) of eluted proteins as well as of reaction mixtures (Input, lower panels) were subjected to SDS-PAGE and Western immunoblotted (IB) with anti-FLAG-HRP, anti-AKAP18-His, anti-PKAc, and anti-phospho-PKA substrate (pPDE3A) antibodies as indicated. Similar amounts of AKAP18δ were immunoprecipitated in the control groups and in reactions incubated with rPKAc. C, as a control, rhPDE3A1 (50 units) was incubated with or without rPKAc (50 units) and with SF21 cell supernatants that contained or did not contain rAKAP18δ. The bar graph summarizes binding of rhPDE3A1-FLAG with rAKAP18δ-His in the absence (control) and presence of rPKAc. The band density of rhPDE3A1 was normalized to that of input rAKAP18δ and calculated as the amounts of rhPDE3A1 bound to rAKAP18δ in the absence (Control, C) or presence of rPKAc; there was an ∼9-fold increase in the association of rhPDE3A1 with AKAP18δ in the presence of rPKAc (*, p < 0.001). Shown are representative blots from three independent experiments.