FIGURE 8.
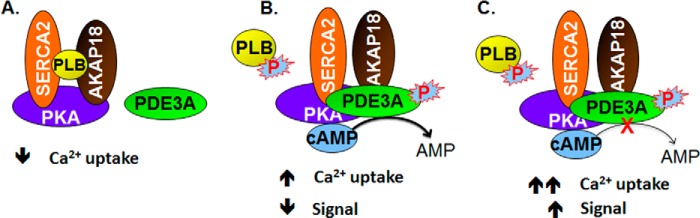
Model of the regulation of SERCA2 activity by cAMP and the AKAP18 and PLB-containing signalosome. A, components of the AKAP18/SERCA2/PLB signalosome are shown. B, in the absence of cAMP, SERCA2 was inhibited by its interaction with PLB. Activation of PKA by cAMP resulted in the phosphorylation of PLB and PDE3A (and, most likely, other molecules in the signalosome). The former dissociates from SERCA2, increasing SERCA2 activity, but the integration of phosphorylated PDE3A into the signalosome limits this effect by increasing hydrolysis of cAMP. PP1 and PP2A in the signalosome would be expected to catalyze the dephosphorylation of PDE3A, PLB, and other PKA substrates and return the SERCA2 complex to its basal state. C, PDE3 inhibition potentiates the effect of cAMP on SERCA2.
