FIGURE 5.
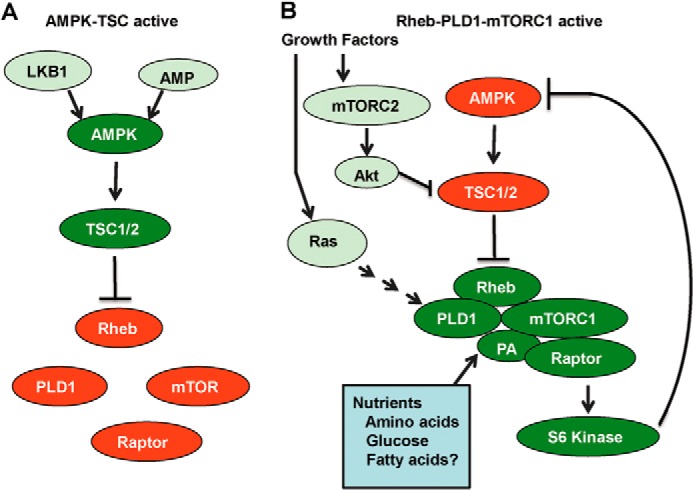
Model for reciprocal regulation of AMPK and PLD. Two scenarios are presented for the regulation of PLD by AMPK and the regulation of AMPK by PLD. A, AMPK is activated by the presence AMP and phosphorylation by LKB1. AMPK then phosphorylates TSC, which stimulates the GAP activity of TSC for Rheb, resulting in the hydrolysis of bound GTP to GDP and inactivation of Rheb. As a consequence, PLD is not activated (19), and the PA needed for stabilization of mTORC1 (21) is not generated, so mTORC1 is inactive. B, Rheb is GTP-bound and promotes the production of PA by PLD and the stabilization of mTORC1. Active mTORC1 then causes suppression of AMPK in a manner that is likely dependent on S6 kinase in that rapamycin has been reported to activate AMPK (30) at concentrations that suppress S6 kinase (38). Other signals promoted by oncogenic stimuli contribute to the activation of this pathway, such as mTORC2-Akt, which suppresses TSC (16), and Ras, which leads to PLD activation (27). It is also important to note that mTOR is an integrator on nutrient and growth factor signals (54), both of which are required for cell cycle progression and proliferation. mTOR requires amino acids (55), glucose (24), and possibly lipids (51) for activity.
