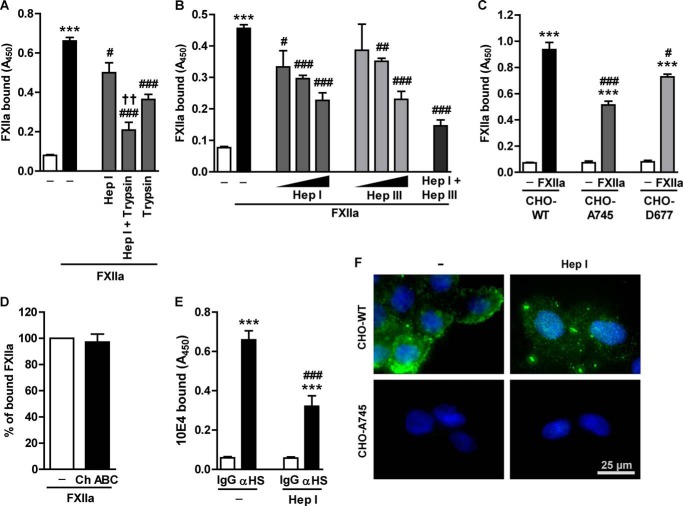
FIGURE 3.
HS mediates interaction of FXIIa with HLF. A, to simultaneously remove HS and surface proteins, HLF were co-incubated with 0.0016 IU of heparinase I (Hep I) and 0.0001% (w/v) trypsin. B, HLF were incubated with 0.0032, 0.0016, or 0.0008 IU of heparinase I; with 0.001, 0.0004, or 0.0002 IU of heparinase III (Hep III); or with both enzymes (0.0016 IU of heparinase I and 0.001 IU of heparinase III) to remove HS from the cell surface. A and B, after incubation with anti-FXII antibodies, FXIIa binding capacity of HLF was analyzed by cell-based ELISA. Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus FXIIa only; ††, p < 0.01 versus Hep I-treated cells. C, wild-type (CHO-WT), GAG-deficient (CHO-A745), or HS-deficient (CHO-D677) CHO cells were treated with FXIIa. Surface accumulation of FXIIa was examined as described above. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ###, p < 0.001 versus CHO-WT cells incubated with FXIIa. D, impact of CS degradation on FXIIa binding. Cells were treated with 0.004 units of chondroitinase ABC (Ch ABC) for 2 h, followed by incubation with FXIIa and measurement of FXIIa binding capacity as described above. Date are given as a percentage of FXIIa binding to chondroitinase ABC-untreated cells (set as 100%) (n = 3). E, effect of heparinase I enzymatic activity on HS surface expression. Cell-based ELISA was performed on the intact HLF and on the cells treated with 0.0016 IU of heparinase I and subsequently incubated with 10E4 antibodies directed against native HS-type GAG (αHS). Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus IgG; ###, p < 0.001 versus anti-HS-type GAG only. F, representative fluorescence microscopy images of CHO-WT and CHO-A745 cells stained with antibodies directed against native heparin sulfate chains (green). Cells were either treated with 0.0016 IU of heparinase I or left untreated (CTRL). Magnification was ×400.