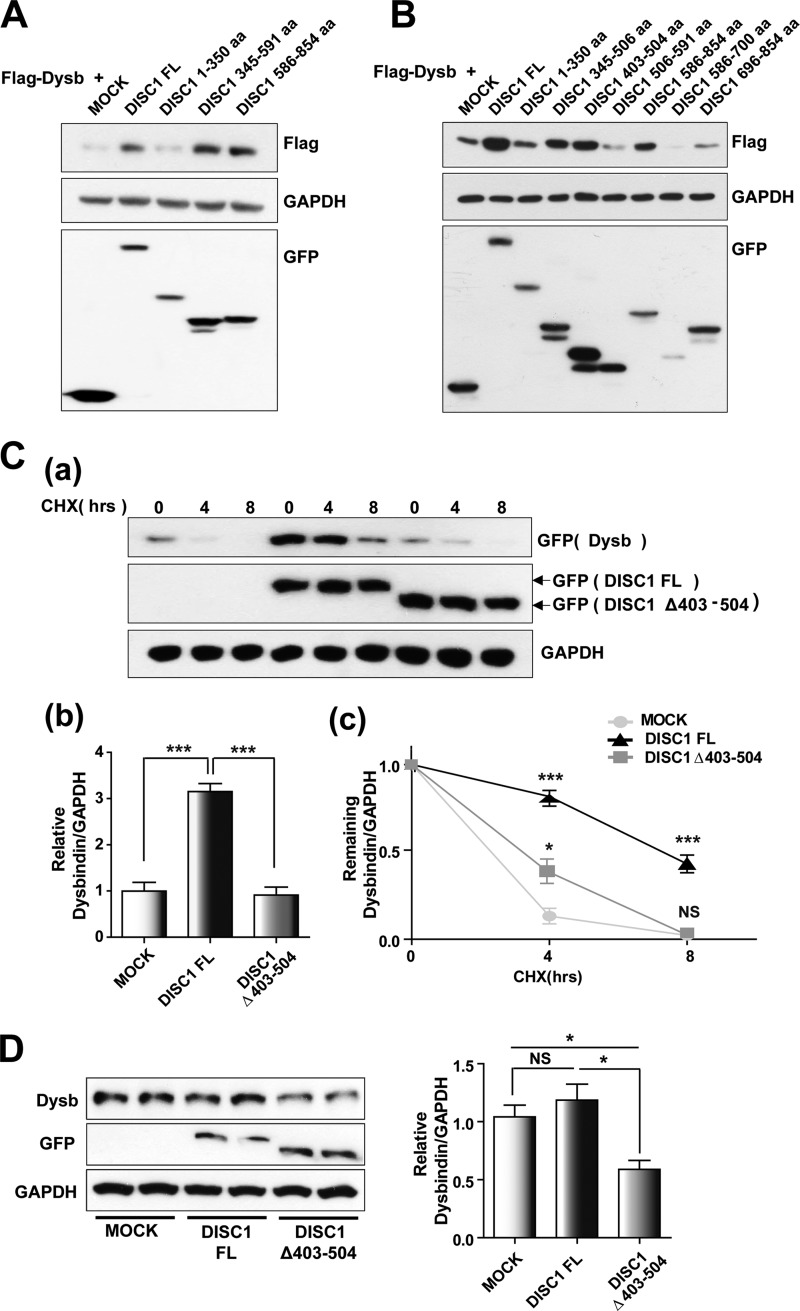
FIGURE 3.
DISC1 enhances dysbindin stability. A, up-regulation of dysbindin is mediated by DISC1 amino acid residues 345–854. FL, full-length; aa, amino acids. B, increased expression of dysbindin (Dysb) upon coexpression with DISC1403–504. 30 h after transfection with the indicated plasmids, cells were harvested for immunoblotting analyses. C, effect of DISC1Δ403–504 coexpression on dysbindin stability. HEK293T cells were treated with 100 μg/ml cycloheximide (CHX) 30 h after cotransfection of EGFP-dysbindin constructs with either full-length DISC1 or DISC1Δ403–504. Dysbindin levels were quantified by immunoblotting. Representative data are shown in a, and the relative dysbindin protein level at time 0 is shown in b. Error bars show mean ± S.E. ***, p < 0.001 by one-way ANOVA with Bonferroni's multiple comparison test (n = 4); NS, not significant. c, the dynamics indicating the remaining protein levels of dysbindin were measured as a ratio of the value at time 0 in the same group. Error bars show mean ± S.E. *, p < 0.05; ***, p < 0.001 versus EGFP-dysbindin with empty vector (MOCK) by one-way ANOVA with Bonferroni's multiple comparison test (n = 4). D, down-regulation of dysbindin protein by overexpression of DISC1Δ403–504. COS7 cells were transfected with either EGFP-DISC1 or EGFP-DISC1Δ403–504 and subjected to immunoblotting. Error bars show mean ± S.E. *, p < 0.05 by one-way ANOVA with Bonferroni's multiple comparison test (n = 3).