Figure 5.
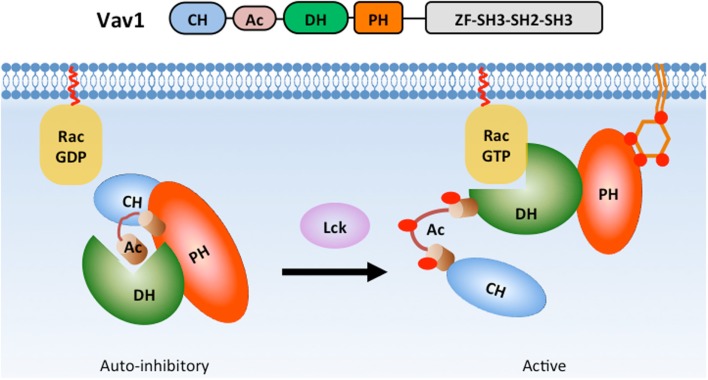
PH domain interactions stabilize Vav1 auto-inhibition in basal state. In the basal state, Vav1 adopts an auto-inhibitory conformation in which the substrate-docking site within the DH domain is blocked by interactions with a helix region from the Ac domain. The interactions between CH, PH, and Ac domains greatly strengthen the auto-inhibitory conformation (left). During T cell activation, phosphorylation of the Ac domain by Lck releases the substrate-docking site and allows GTPase binding (right).