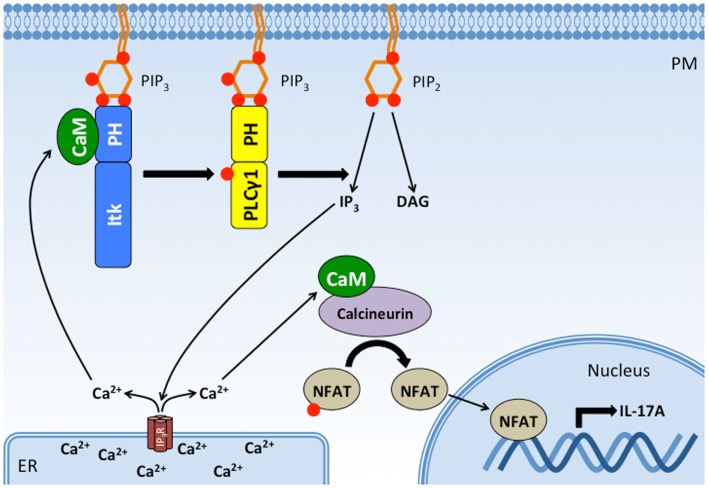
Figure 6.
CaM binds the Itk PH domain in a positive feedback loop that potentiates Itk activity, intracellular Ca2+ release, and IL-17A production. Binding of Itk to PIP3 promotes Itk activation and the subsequent phosphorylation and activation of PLC γ1. PLCγ1 cleaves PIP2 to produce DAG and IP3, which binds IP3 receptors on the ER. The IP3 receptor is a ligand-gated Ca2+ channel, and its activation increases Ca2+ levels in the cytosol. Increased cytosolic Ca2+ activates CaM, which has at least two effects on T cell activation: (1) Ca2+/CaM binds to Itk’s PH domain, enhancing its interaction with PIP3 and Itk activity. (2) Ca2+/CaM binds to and activates calcineurin, a phophatase that dephosphorylates NFAT, allowing NFAT translocation to the nucleus where it drives the transcription of IL-17A. Thus, CaM binding to Itk’s PH domain completes a positive feedback loop that potentiates the downstream effects of Itk. PM, plasma membrane; ER, endoplasmic reticulum; Itk, IL-2-inducible tyrosine/T cell kinase; PLCγ1, phospholipase C gamma 1; CaM, calmodulin; NFAT, nuclear factor of activated T cells; IP3R, IP3 receptor.