FIGURE 1.
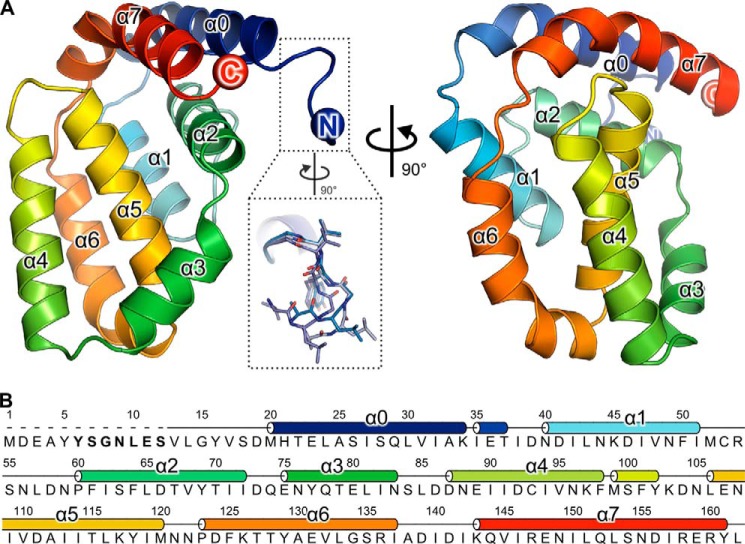
A49 adopts a Bcl-2-like fold. A, the structure of A49 Δ12 is shown in two orthogonal views as a ribbon colored from blue (N terminus) to red (C terminus). The inset shows the alternative conformations of residues 13–17 observed, highlighting the mobility of this region. In crystals of A49 grown from full-length protein, no density was observed for these residues. B, sequence of VACV WR A49. The secondary structure is shown above the sequence, and residues thought to interact with the β-propeller domain of β-TrCP are in boldface type.