FIGURE 2.
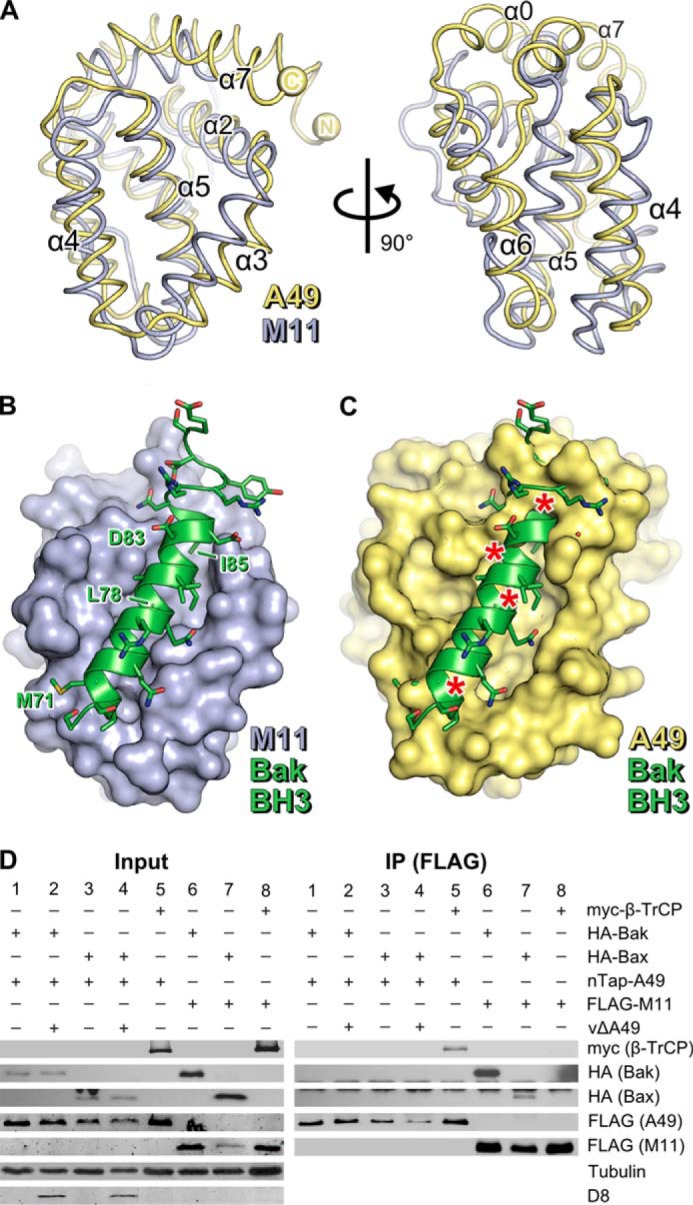
A49 lacks a surface groove and does not bind BH3 peptides. A, the structures of MYXV M11 (blue ribbons, PDB 2JBY (14)) and VACV A49 (yellow ribbons) are shown, superposed, in two orthogonal views. Selected helices are labeled. B, the structure of the human Bak BH3 peptide (green ribbon and side chains) bound to M11 (blue molecular surface) is shown (PDB 2JBY (14)). C, model of A49 in complex with the human Bak BH3 peptide generated by superposing A49 onto the M11-Bak complex (14). Asterisks denote significant clashes. D, unlike M11, A49 does not co-immunoprecipitate with human Bak or Bax. HEK293T cells were transfected with Myc-β-TrCP (lanes 5 and 8), HA-Bak (lanes 1, 2, and 6), or HA-Bax (lanes 3, 4, and 7) and nTAP-A49 (lanes 1–5) or FLAG-M11 (lanes 6–8). After 24 h, cells were infected with vΔA49 at 5 pfu/cell (lanes 2 and 4) or mock-infected (lanes 1, 3, and 5–8). Cells were lysed 6 h after infection, and lysates were immunoprecipitated (IP) with an anti-FLAG matrix before immunoblotting using the antibodies specified. Tubulin served as a loading control, and D8, a VACV envelope protein, served as a positive marker of infection.
