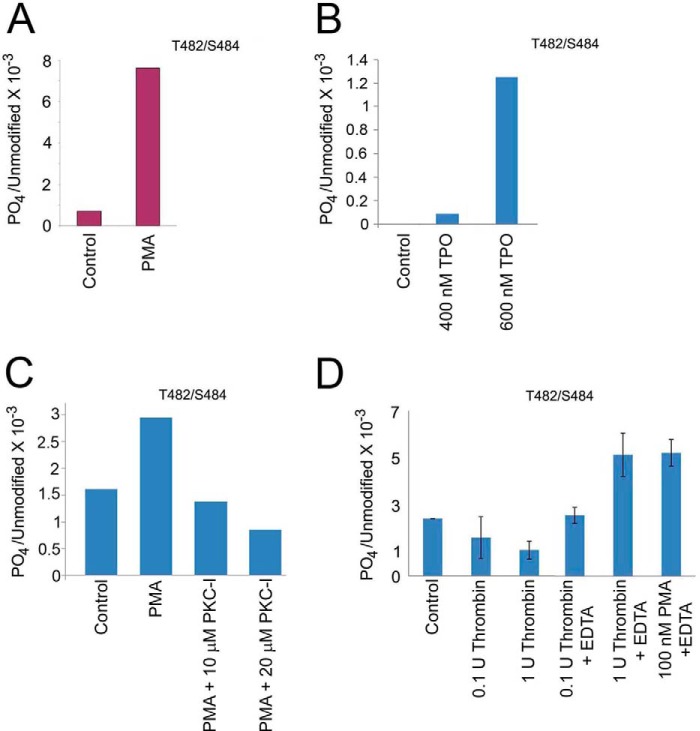
FIGURE 6.
Kindlin-3 is phosphorylated in HEL cells and platelets upon agonist stimulation. A, HEL cells were treated with PMA, and endogenous kindlin-3 was immunoprecipitated with kindlin-3 antibodies. The 72-kDa kindlin-3 band was excised from the gel and subjected to mass spectrometry sequencing. Thr482 and/or Ser484 were identified as a phosphosite in three independent experiments. B, HEL cells were treated with TPO, and endogenous kindlin-3 was immunoprecipitated with kindlin-3 antibodies. The 72-kDa kindlin-3 band was excised from the gel and subjected to mass spectrometry sequencing. Thr482 and/or Ser484 were identified as a phosphosite in two independent experiments. C, HEL cells were treated with PMA in the absence or presence of membrane-permeable PKC inhibitor, and endogenous kindlin-3 was immunoprecipitated with kindlin-3 antibodies. The 72-kDa kindlin-3 band was excised from the gel and subjected to mass spectrometry sequencing. Thr482 and/or Ser484 were identified as a phosphosite in two independent experiments. D, human platelets were treated with thrombin or PMA, and endogenous kindlin-3 was immunoprecipitated with kindlin-3 antibodies. The 72-kDa band was excised from the gels and subjected to mass spectrometry sequencing. Mass analysis of the spectra suggested a single phosphorylation site in kindlin-3 from both cell types, but the identity of the site, Thr482 or Ser484, could not be distinguished. These phosphosites reside in a hyper-variable region of kindlin-3 region, i.e. it is not present in either kindlin-1 or kindlin-2.