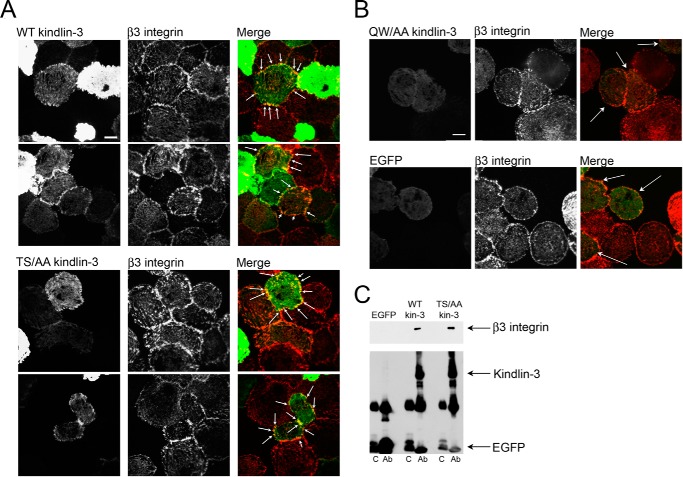
FIGURE 8.
Kindlin-3 phosphomutant (S482A/T484A (S482T484/AA) colocalizes with β3 integrin in focal adhesions as assessed by TIRF and binds to β3 integrin. A and B, Hel cells were transiently transfected with plasmids encoding EGFP alone and EGFP-kindlin-3 constructs. Transfected HEL cells were treated with PMA and allowed to adhere to fibrinogen for 1 h. The adherent cells were fixed and stained with β3 integrin antibody followed with Alexa 568-conjugated anti-mouse secondary antibody. Kindlin-3 distribution was visualized with EGFP fluorescence. A, wild-type and phosphomutant kindlin-3 colocalized with integrins in focal adhesions (indicated with arrows). B, Q597A/W598A (QW/AA) kindlin-3 (mutation of the primary integrin-binding site) and EGFP alone were absent from β3-containing focal adhesions (transfected cells indicated with arrows). Bar, 20 μm. C, lysates of CHO-A5 cells transfected with EGFP-tagged wild-type kindlin-3, S482A/T484A kindlin-3, or EGFP alone were used for coimmunoprecipitation assays. After incubating with A/G-agarose and EGFP antibody (Ab), β3 integrin bound to kindlin-3 constructs was evaluated by SDS-PAGE and Western blotting using anti-β3 integrin antibody. Kindlin-3 and EGFP levels in immunoprecipitates are also shown as detected with an EGFP antibody. C, control.