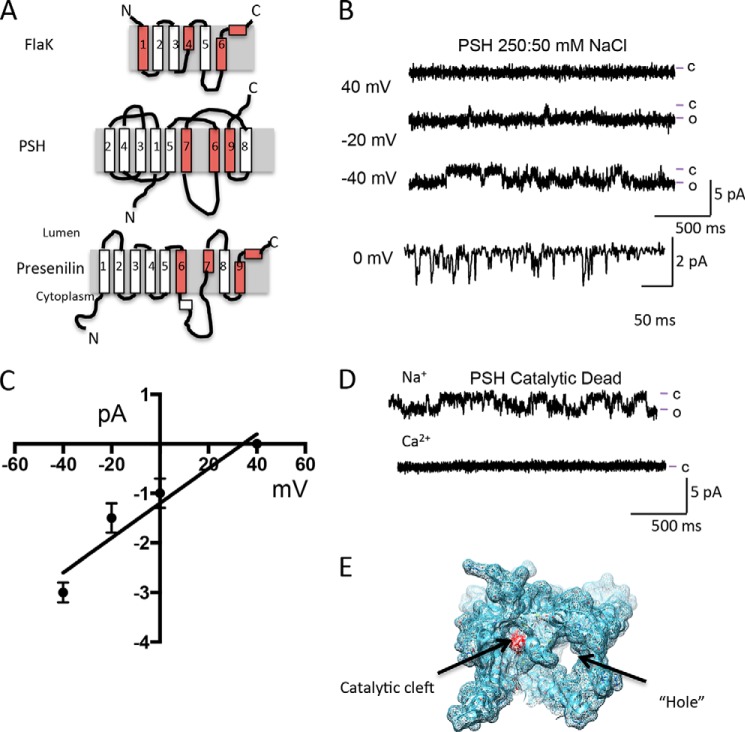
FIGURE 1.
The GxGD protease PSH forms a channel. A, cartoon depiction of PSH, FlaK, and presenilin. Helices containing residues critical for protease activity and formation of the catalytic cleft are colored red. B, reconstitution of PSH into the lipid bilayer results in currents. Lines to the side of the traces represent the open (o) and closed (c) current levels. Current traces shown were filtered at 250 Hz. C, current-voltage relationship of PSH with 250 mm NaCl on the cis side and 50 mm NaCl on the trans side suggests that PSH is a monovalent cation selective ion channel. Current amplitude values are calculated from at least 200 openings at each voltage from at least three experiments using at least two different protein preparations. D, Na+ currents are still present in a catalytic dead mutant version of PSH at 0 mV. Note that there are no current openings observed with Ca2+ as the current carrier. E, depiction of the hole and the catalytic cleft in PSH (PDB: 4HYG and 4HYD).