FIGURE 3.
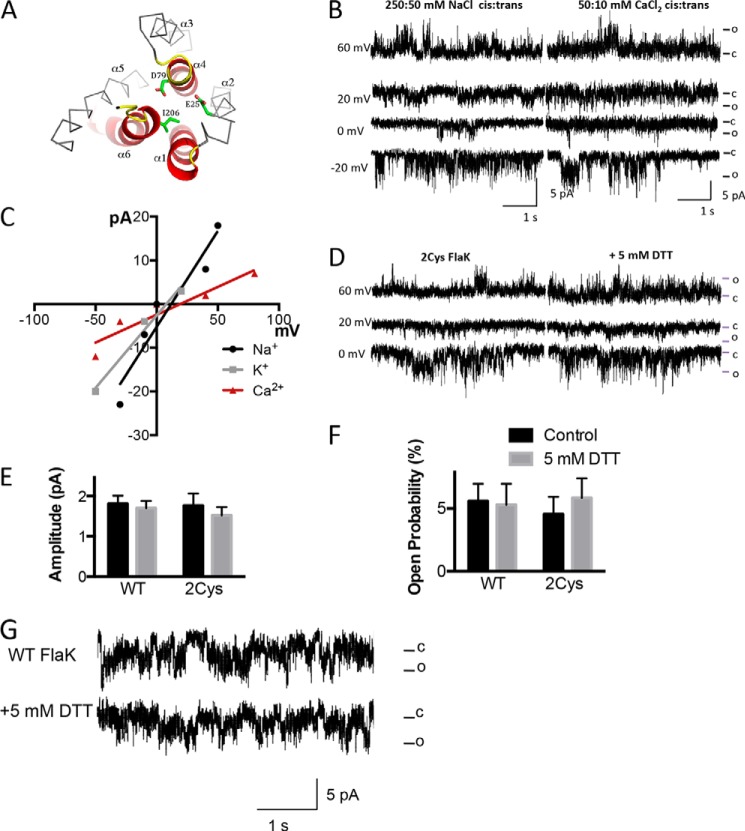
Catalytic dead FlaK variants D79N and 2Cys FlaK have cation channel activity. A, schematic diagram of the mutations made to FlaK to diminish its catalytic activity. In this top view cartoon, without the soluble domain for clarity, the catalytic critical core structure is shown in red. The highly conserved motifs contributing to the catalytic site are in yellow. The D79N, E25C, and I206C mutations made in this work are highlighted in green in a stick model. The helices are labeled α1–6. B, reconstitution of D79N FlaK into the lipid bilayer results in Na+ currents similar to WT FlaK (left traces). D79N FlaK conducts Ca2+ (right traces). Currents were obtained with 50:10 mm Ca2Cl cis:trans. C, current-voltage relationship of D79N FlaK with Na+, K+ or Ca2+ as charge carrier. Each point is the average of >100 channel openings from three different protein preparations. D, insertion of 2Cys FlaK into the lipid bilayer resulted in monovalent cation channel activity (left traces). Breaking the crosslinking disulfide bonds with DTT did not affect channel activity (right traces). Experiments were performed with 250:50 mm NaCl cis:trans. E and F, 5 mm DTT does not have a significant effect on Na+ current amplitude (E) or the Na+ current open probability (F) of the channel formed by WT or 2Cys-FlaK (n = 3–5). Error bars represent the S.E. G, WT FlaK inserted into the bilayer was not affected by the addition of 5 mm DTT. Traces are taken from experiments containing 250:50 mm NaCl cis:trans, at 0 mV holding potential.