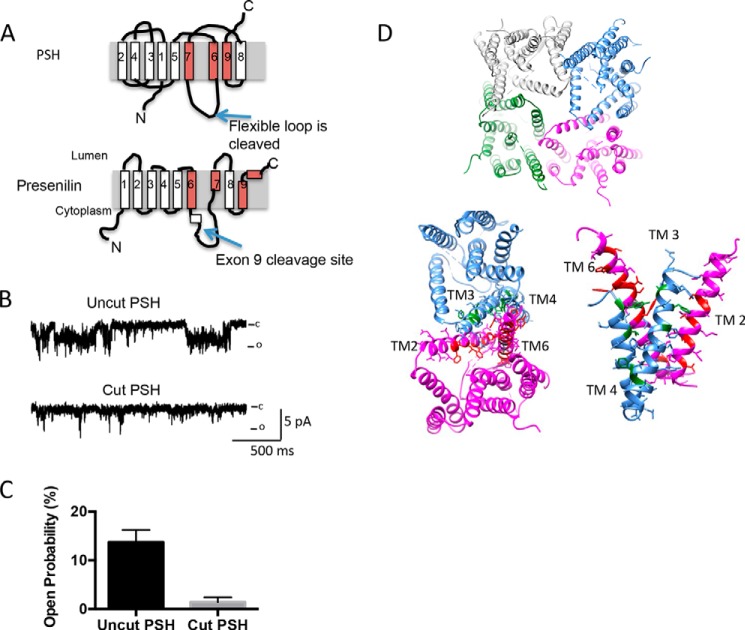
FIGURE 5.
The cutting of PSH largely abolishes channel activity. A, schematic diagram of the cut sites. B, insertion of cut PSH (which still is capable of catalytic activity) into the lipid bilayer largely diminishes activity with 250:50 mm NaCl at 0 mV. C, analysis of PSH activity at 0 mV with 250:50 mm NaCl at 0 mV. Analysis is from three different experiments; examining bursts of activity of 30–90 s duration. Error bars represent S.E. D, depiction of how four PSH molecules can arrange to form a channel-like structure (top). Depiction of the interface between two neighboring PSH molecules (bottom left). The corresponding residues in presenilin that are known to be associated with FAD are highlighted in either red or green. These residues were selected from an alignment of PSH and presenilin previously published (9). A side-on view of the interface between two neighboring PSH molecules is shown (bottom right).