FIGURE 2.
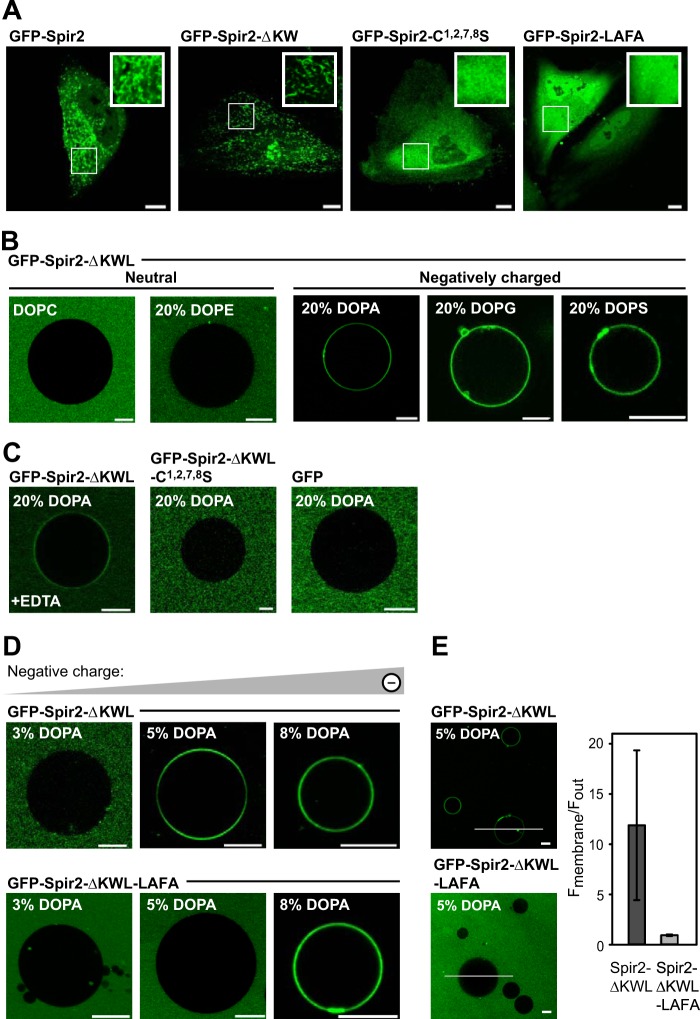
The Spir-2 FYVE-type zinc finger domain mediates membrane binding. A, confocal images showing the subcellular distribution of GFP-tagged Spir-2 constructs in HeLa cells. Wild-type Spir-2 and the deletion construct containing a functional FYVE-type domain, Spir-2-ΔKW, accumulate at intracellular vesicular membranes, whereas the mutants C1,2,7,8S and LAFA are evenly distributed in the cytoplasm. B–E, binding of recombinant GFP-tagged Spir-2 constructs at the membrane of GUVs composed of DOPC supplemented by the indicated differently charged lipid species (%). B, GFP-Spir-2-ΔKWL binds to negatively charged GUVs (DOPA, 1,2-dioleoyl-sn-glycero-3-phosphatidylglycerol (DOPG), or 1,2-dioleoyl-sn-glycero-3-phosphatidylserine (DOPS)) but not to neutral GUVs (DOPC and 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE)). C, membrane binding is reduced in the presence of 50 mm EDTA, which chelates the positively charged zinc ions. Disintegration of the FYVE-type domain (C1,2,7,8S) or its removal (free GFP) prevents membrane binding. D, the wild-type Spir-2 FYVE-type domain requires a lower content of negatively charged lipids to mediate binding than the less hydrophobic LAFA mutant. E, quantification of membrane-associated fluorescence with line profiles. Scale bars represent 10 μm. Error bars represent S.D.