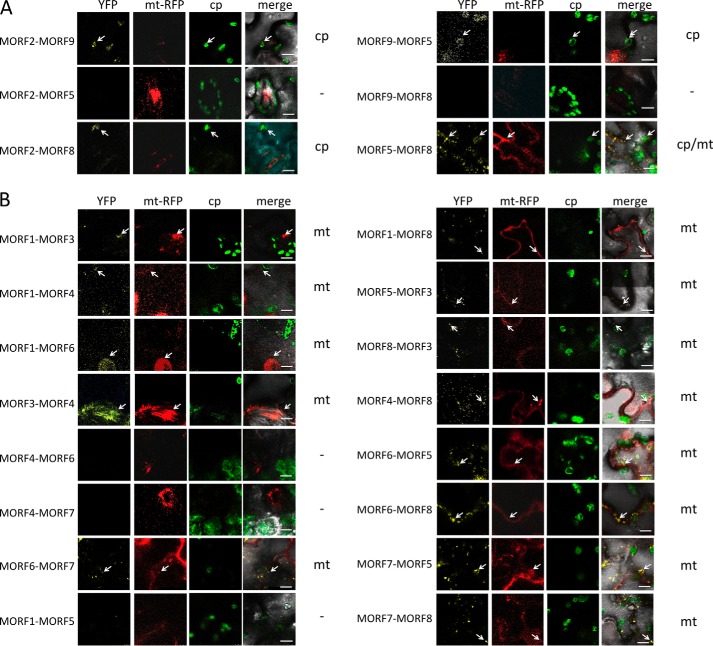
FIGURE 5.
MORF-MORF protein heteromer formation is observed in BiFC complementation assays. A, interactions of plastid-located MORF proteins. MORF2 and MORF9 proteins connect to heteromers, as indicated by the reconstituted YFP fluorescence from the N- and C-terminal YFP parts fused to the respective MORF reading frame. The MORF protein given first is fused to YFP-N, and the second is fused to YFP-C, respectively. MORF2 and MORF9 proteins are located in the plastid, and MORF5 and MORF8 proteins are dual-targeted to plastids and to mitochondria. MORF5 yields no detectable bimolecular interaction with MORF2, but connects with MORF8 in both organelles and with MORF9 in the plastid. The MORF8 protein contacts the MORF2 protein, but yields no signal with MORF9. Locations of the YFP signals are indicated on the right; the dash indicates that no YFP signal is detected. Arrows point out exemplary signals. B, interactions of mitochondrially located MORF proteins. Details of the various MORF-MORF protein heteromer connections are discussed under “Results” and in Figs. 6 and 7.